Imaging markers of response to combined BRAF and MEK inhibition in BRAF mutated vemurafenib-sensitive and resistant melanomas
- PMID: 29682188
- PMCID: PMC5908289
- DOI: 10.18632/oncotarget.24709
Imaging markers of response to combined BRAF and MEK inhibition in BRAF mutated vemurafenib-sensitive and resistant melanomas
Abstract
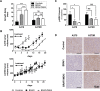
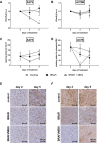
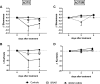
A majority of patients with a V600x melanoma respond quickly to BRAF/MEK inhibition (BRAFi/MEKi) and have an obvious clinical benefit. Nearly all the patients after this initial phase will develop resistance. Therefore, non-invasive early markers of response/non-response are needed in order to identify those patients who, due to intrinsic or acquired resistance, do not respond to treatment and would be eligible for alternative treatments. The aim of this study was to investigate the value of magnetic resonance spectroscopy (1H-MRS) of choline and diffusion-weighted magnetic resonance imaging (DW-MRI) as early markers of response to BRAF inhibition (BRAFi) with vemurafenib alone or in combination with MEK inhibition (MEKi) with trametinib, in BRAFi-sensitive and BRAFi-resistant melanoma xenografts. Tumor response was significantly improved by the combination of BRAFi and MEKi, compared to BRAFi alone, only in sensitive xenografts; thus indicating that vemurafenib-resistant A375R xenografts were cross-resistant to the inhibition of MEK, as confirmed by immunohistochemistry analysis for phosphorylated ERK. In vivo1H-MRS showed that in sensitive melanoma xenografts, a significant blockage of ERK phosphorylation, but not a decrease in cell proliferation, was required to affect total choline (tCho) levels, thus suggesting that tCho could serve as a pharmacodynamic (PD) marker for agents targeting the MAPK cascade. In addition, early effects of the combination therapy on tumor cellularity could be detected via DW-MRI. In particular, skewness and kurtosis of the apparent diffusion coefficient (ADC) distribution may be useful to detect changes in the diffusional heterogeneity that might not affect the global ADC value.
Keywords: BRAF/MEK inhibitors; choline spectroscopy; diffusion-weighted MRI; melanoma; tumor response.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity.Onco Targets Ther. 2013 Nov 28;6:1649-58. doi: 10.2147/OTT.S52552. eCollection 2013. Onco Targets Ther. 2013. PMID: 24348046 Free PMC article.
-
Adverse effects of systemic advanced melanoma therapies-do BRAF/MEK inhibitors increase the incidence of mesenteric panniculitis?Eur Radiol. 2025 May 1. doi: 10.1007/s00330-025-11642-w. Online ahead of print. Eur Radiol. 2025. PMID: 40310541
-
Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma.J Am Acad Dermatol. 2014 Dec;71(6):1102-1109.e1. doi: 10.1016/j.jaad.2014.09.002. Epub 2014 Oct 16. J Am Acad Dermatol. 2014. PMID: 25440439 Free PMC article.
-
Combination Treatment of Patients with BRAF-Mutant Melanoma: A New Standard of Care.BioDrugs. 2017 Feb;31(1):51-61. doi: 10.1007/s40259-016-0208-z. BioDrugs. 2017. PMID: 28058658 Review.
-
BRAF and MEK inhibition in melanoma.Expert Opin Drug Saf. 2015 Apr;14(4):559-70. doi: 10.1517/14740338.2015.1011618. Epub 2015 Feb 4. Expert Opin Drug Saf. 2015. PMID: 25648338 Review.
Cited by
-
Development of a Hybrid-Imaging-Based Prognostic Index for Metastasized-Melanoma Patients in Whole-Body 18F-FDG PET/CT and PET/MRI Data.Diagnostics (Basel). 2022 Aug 30;12(9):2102. doi: 10.3390/diagnostics12092102. Diagnostics (Basel). 2022. PMID: 36140504 Free PMC article.
-
EGFR/uPAR interaction as druggable target to overcome vemurafenib acquired resistance in melanoma cells.EBioMedicine. 2019 Jan;39:194-206. doi: 10.1016/j.ebiom.2018.12.024. Epub 2019 Jan 2. EBioMedicine. 2019. PMID: 30611716 Free PMC article.
-
Probing metabolic alterations in breast cancer in response to molecular inhibitors with Raman spectroscopy and validated with mass spectrometry.Chem Sci. 2020 Aug 20;11(36):9863-9874. doi: 10.1039/d0sc02221g. Chem Sci. 2020. PMID: 34094246 Free PMC article.
-
Metabolic imaging using hyperpolarized 13 C-pyruvate to assess sensitivity to the B-Raf inhibitor vemurafenib in melanoma cells and xenografts.J Cell Mol Med. 2020 Jan;24(2):1934-1944. doi: 10.1111/jcmm.14890. Epub 2019 Dec 13. J Cell Mol Med. 2020. PMID: 31833658 Free PMC article.
References
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review. 2017. pp. 1975–2014.
-
- Lui P, Cashin R, Machado M, Hemels M, Corey-Lisle PK, Einarson TR. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treat Rev. 2007;33:665–80. https://doi.org/10.1016/j.ctrv.2007.06.004 - DOI - PubMed
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. https://doi.org/10.1038/nature00766 - DOI - PubMed
-
- Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14:463–82. https://doi.org/10.1038/nrclinonc.2017.43 - DOI - PubMed
-
- Kudchadkar R, Paraiso KH, Smalley KS. Targeting mutant BRAF in melanoma: current status and future development of combination therapy strategies. Cancer J. 2012;18:124–31. https://doi.org/10.1097/PPO.0b013e31824b436e - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

