Testosterone metabolites inhibit proliferation of castration- and therapy-resistant prostate cancer
- PMID: 29682196
- PMCID: PMC5908297
- DOI: 10.18632/oncotarget.24763
Testosterone metabolites inhibit proliferation of castration- and therapy-resistant prostate cancer
Abstract
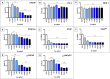
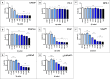
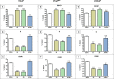
Novel treatments for castration-resistant prostate cancer (CRPC) such as abiraterone acetate (AA) or enzalutamide effectively target the androgen pathway to arrest aberrant signalling and cell proliferation. Testosterone is able to inhibit tumour cell growth in CRPC. Estrogen receptor-beta (ERβ) binds the testosterone-metabolites 3β-androstanediol and 3α-androstanediol in parallel to the canonical estradiol. In the prostate it is widely accepted that ERβ regulates estrogen signalling, mediating anti-proliferative effects. We used the prostate cancer cell lines LNCaP, PC-3, VCaP, and the non-neoplastic BPH-1. VCaP cells were treated with 1 nmol/L testosterone over 20 passages, yielding the cell line VCaPrev, sensitive to hormone therapies. In contrast, LNCaP cells were grown for more than 100 passages yielding a high passage therapy resistant cell line (hiPLNCaP). VCaP and hiPLNCaP cell lines were treated with 5 μmol/L AA for more than 20 passages, respectively, generating the AA-tolerant-subtypes VCaPAA and hiPLNCaPAA. Cell lines were treated with testosterone, dihydrotestosterone (DHT), R1881, and the androgen-metabolites 3β-androstanediol and 3α-androstanediol. 3β-androstanediol or 3α-androstanediol significantly reduced proliferation in all cell lines except the BPH-1 and androgen receptor-negative PC-3 and markedly downregulated AR and estrogen receptor alpha (ERα). Whereas ERβ expression was increased in all cell lines except BPH-1 or PC-3. In summary, 3β-adiol or 3α-adiol, as well as DHT and R1881, significantly reduced tumour cell growth in CRPC cells. Thus, these compounds represent novel potential therapeutic approaches to overcome drug-resistance in CRPC, especially with regard to AR-V7 function in therapy resistance. Furthermore, these data confirm the tumour suppressor properties of ERβ in CRPC.
Keywords: 3a-androstendiol; 3b-androstendiol; AKR1C1; AKR1C2 and AKR1C3; castration resistant prostate cancer.
Conflict of interest statement
CONFLICTS OF INTEREST P.T. received consulting from Janssen-Cilag.
Figures


References
-
- Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–52. https://doi.org/10.1016/j.ejca.2010.09.013 - DOI - PubMed
-
- Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. https://doi.org/10.1016/j.eururo.2012.02.054 - DOI - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79. https://doi.org/10.1016/j.eururo.2013.11.002 - DOI - PubMed
-
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–46. https://doi.org/10.1016/S0140-6736(10)60172-9 - DOI - PMC - PubMed
-
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, et al. AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. https://doi.org/10.1056/NEJMoa1207506 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

