Ferric citrate and ferric EDTA but not ferrous sulfate drive amphiregulin-mediated activation of the MAP kinase ERK in gut epithelial cancer cells
- PMID: 29682205
- PMCID: PMC5908306
- DOI: 10.18632/oncotarget.24899
Ferric citrate and ferric EDTA but not ferrous sulfate drive amphiregulin-mediated activation of the MAP kinase ERK in gut epithelial cancer cells
Abstract
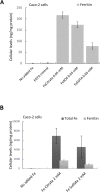
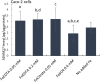
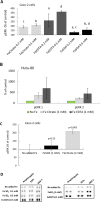
Ferric chelates may be used as oral iron supplements or phosphate binders but both ferric citrate and ferric EDTA have been shown to promote tumor burden in murine models of colon cancer. Here we studied their effects on cancer cell growth, at typical supplemental iron levels encountered in the gastrointestinal tract (0.01-0.2 mM). Caco-2 and/or Hutu-80 cells were exposed to these forms of chelated iron or to ferrous sulfate and outcomes were assessed using cell proliferation assays, proteome profiler arrays, western blot, and ELISA. Ferric EDTA and ferric citrate increased cellular levels of the onco-protein amphiregulin and its receptor (EGFr) which in turn stimulated the activation of the MAP kinase ERK. Simultaneously, the expression of the negative Wnt regulator, DKK-1, increased suggesting that cell proliferation through the Wnt pathway may be less pronounced in the presence of ferric EDTA and ferric citrate, unlike for ferrous sulfate. Moreover, ferrous sulfate did not increase levels of cellular amphiregulin or EGFr. We conclude that specific iron compounds affect cell signaling differently and some may increase the risk of colon cancer advancement in an amphiregulin-dependent fashion. Further scrutiny of safe oral iron use is merited.
Keywords: amphiregulin; ferric EDTA; ferric citrate; iron; pERK.
Conflict of interest statement
CONFLICTS OF INTEREST DIAP, NF, and JJP are inventors of MRC-owned technologies for iron supplementation or phosphate binding other than those described herein and for which they have financially benefitted via consultancy and MRC Awards to Inventor Scheme. The authors could also receive future awards to inventors for these technologies. Notwithstanding, the authors declare no conflict of interest.
Figures



Similar articles
-
Differential Effects of Iron Chelates vs. Iron Salts on Induction of Pro-Oncogenic Amphiregulin and Pro-Inflammatory COX-2 in Human Intestinal Adenocarcinoma Cell Lines.Int J Mol Sci. 2023 Mar 14;24(6):5507. doi: 10.3390/ijms24065507. Int J Mol Sci. 2023. PMID: 36982582 Free PMC article.
-
Effect of Ferric Citrate versus Ferrous Sulfate on Iron and Phosphate Parameters in Patients with Iron Deficiency and CKD: A Randomized Trial.Clin J Am Soc Nephrol. 2020 Sep 7;15(9):1251-1258. doi: 10.2215/CJN.15291219. Epub 2020 Jul 21. Clin J Am Soc Nephrol. 2020. PMID: 32694162 Free PMC article. Clinical Trial.
-
Inhibition of iron uptake from iron salts and chelates by divalent metal cations in intestinal epithelial cells.J Agric Food Chem. 2005 Jan 12;53(1):132-6. doi: 10.1021/jf049255c. J Agric Food Chem. 2005. PMID: 15631519
-
Ferric carboxymaltose: a review of its use in iron-deficiency anaemia.Drugs. 2009;69(6):739-56. doi: 10.2165/00003495-200969060-00007. Drugs. 2009. PMID: 19405553 Review.
-
[Consideration of the Characteristics of Oral Iron Preparations from the Viewpoint of the Mechanism of Nausea and Vomiting].Yakugaku Zasshi. 2023;143(7):599-606. doi: 10.1248/yakushi.23-00057. Yakugaku Zasshi. 2023. PMID: 37394455 Review. Japanese.
Cited by
-
Iron in the Tumor Microenvironment-Connecting the Dots.Front Oncol. 2018 Nov 26;8:549. doi: 10.3389/fonc.2018.00549. eCollection 2018. Front Oncol. 2018. PMID: 30534534 Free PMC article. Review.
-
Differential Effects of Iron Chelates vs. Iron Salts on Induction of Pro-Oncogenic Amphiregulin and Pro-Inflammatory COX-2 in Human Intestinal Adenocarcinoma Cell Lines.Int J Mol Sci. 2023 Mar 14;24(6):5507. doi: 10.3390/ijms24065507. Int J Mol Sci. 2023. PMID: 36982582 Free PMC article.
-
Ferric citrate for the treatment of hyperphosphatemia and iron deficiency anaemia in patients with NDD-CKD: a systematic review and meta-analysis.Front Pharmacol. 2024 Mar 7;15:1285012. doi: 10.3389/fphar.2024.1285012. eCollection 2024. Front Pharmacol. 2024. PMID: 38515853 Free PMC article. Review.
-
The Antagonistic and Synergistic Role of Fe3+ Compounds in Chemo- and Electrochemotherapy in Human Colon Cancer In Vitro.Pharmaceuticals (Basel). 2024 May 17;17(5):651. doi: 10.3390/ph17050651. Pharmaceuticals (Basel). 2024. PMID: 38794222 Free PMC article.
-
The food additive EDTA aggravates colitis and colon carcinogenesis in mouse models.Sci Rep. 2021 Mar 4;11(1):5188. doi: 10.1038/s41598-021-84571-5. Sci Rep. 2021. PMID: 33664327 Free PMC article.
References
-
- Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87:98–104. - PubMed
-
- Floege J, Covic AC, Ketteler M, Rastogi A, Chong EM, Gaillard S, Lisk LJ, Sprague SM, Group PAS. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86:638–47. https://doi.org/10.1038/ki.2014.58 - DOI - PMC - PubMed
-
- Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36:478–87. https://doi.org/10.1159/000344008 - DOI - PubMed
-
- Liao J, Seril DN, Yang AL, Lu GG, Yang GY. Inhibition of chronic ulcerative colitis associated adenocarcinoma development in mice by inositol compounds. Carcinogenesis. 2007;28:446–54. - PubMed
-
- Radulescu S, Brookes MJ, Salgueiro P, Ridgway RA, McGhee E, Anderson K, Ford SJ, Stones DH, Iqbal TH, Tselepis C, Sansom OJ. Luminal iron levels govern intestinal tumorigenesis after apc loss in vivo. Cell Rep. 2016;17:2805–7. https://doi.org/10.1016/j.celrep.2016.10.028 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

