Effects of interfacial micromotions on vitality and differentiation of human osteoblasts
- PMID: 29682285
- PMCID: PMC5895940
- DOI: 10.1302/2046-3758.72.BJR-2017-0228.R1
Effects of interfacial micromotions on vitality and differentiation of human osteoblasts
Abstract
Objectives: Enhanced micromotions between the implant and surrounding bone can impair osseointegration, resulting in fibrous encapsulation and aseptic loosening of the implant. Since the effect of micromotions on human bone cells is sparsely investigated, an in vitro system, which allows application of micromotions on bone cells and subsequent investigation of bone cell activity, was developed.
Methods: Micromotions ranging from 25 µm to 100 µm were applied as sine or triangle signal with 1 Hz frequency to human osteoblasts seeded on collagen scaffolds. Micromotions were applied for six hours per day over three days. During the micromotions, a static pressure of 527 Pa was exerted on the cells by Ti6Al4V cylinders. Osteoblasts loaded with Ti6Al4V cylinders and unloaded osteoblasts without micromotions served as controls. Subsequently, cell viability, expression of the osteogenic markers collagen type I, alkaline phosphatase, and osteocalcin, as well as gene expression of osteoprotegerin, receptor activator of NF-κB ligand, matrix metalloproteinase-1, and tissue inhibitor of metalloproteinase-1, were investigated.
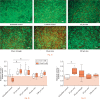
Results: Live and dead cell numbers were higher after 25 µm sine and 50 µm triangle micromotions compared with loaded controls. Collagen type I synthesis was downregulated in respective samples. The metabolic activity and osteocalcin expression level were higher in samples treated with 25 µm micromotions compared with the loaded controls. Furthermore, static loading and micromotions decreased the osteoprotegerin/receptor activator of NF-κB ligand ratio.
Conclusion: Our system enables investigation of the behaviour of bone cells at the bone-implant interface under shear stress induced by micromotions. We could demonstrate that micromotions applied under static pressure conditions have a significant impact on the activity of osteoblasts seeded on collagen scaffolds. In future studies, higher mechanical stress will be applied and different implant surface structures will be considered.Cite this article: J. Ziebart, S. Fan, C. Schulze, P. W. Kämmerer, R. Bader, A. Jonitz-Heincke. Effects of interfacial micromotions on vitality and differentiation of human osteoblasts. Bone Joint Res 2018;7:187-195. DOI: 10.1302/2046-3758.72.BJR-2017-0228.R1.
Keywords: Endoprosthesis; Micromotion; Osseointegration; Osteoblast; Osteoblast differentiation.
Conflict of interest statement
Conflicts of Interest Statement: The authors state no conflicts of interest.
Figures




References
-
- Takenaga RK, Callaghan JJ, Bedard NA, et al. Cementless total hip arthroplasty in patients fifty years of age or younger: a minimum ten-year follow-up. J Bone Joint Surg [Am] 2012;94-A:2153-2159. - PubMed
-
- Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent 1983;50:399-410. - PubMed
-
- Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. Biology of implant osseointegration. J Musculoskelet Neuronal Interact 2009;9:61-71. - PubMed
-
- Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J 2016;98-B(Suppl A):6-9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

