Safety and pharmacokinetics of dolutegravir in HIV-positive pregnant women: a systematic review
- PMID: 29682297
- PMCID: PMC5892677
- DOI: 10.1016/S2055-6640(20)30247-8
Safety and pharmacokinetics of dolutegravir in HIV-positive pregnant women: a systematic review
Abstract
Background: The integrase strand transfer inhibitor dolutegravir (DTG) is being introduced into low- and middle-income countries (LMICs) as an alternative to first-line treatment with non-nucleoside reverse transcriptase inhibitors. However, DTG is not yet widely recommended for use in pregnant women. The aim of this systematic review was to analyse all available data on birth outcomes and congenital anomalies in the infants of pregnant women treated with DTG.
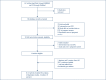
Methods: A PubMed and Embase search was conducted using the terms "dolutegravir" or "DTG" and "pregnancy" or "pregnant" from the earliest available date on the database to 26 July 2017. Any reports involving women who were pregnant, HIV positive and taking DTG were included. The percentage of pregnant women with adverse birth outcomes or congenital anomalies in their infants after taking dolutegravir was compared with five historical control databases.
Results: There were six databases included in the main analysis of birth outcomes and congenital anomalies, with a total of 1200 pregnant women. The percentage of pregnant women taking DTG with adverse birth outcomes and congenital abnormalities was similar to results from historical control studies of HIV-positive women. However, there was significant heterogeneity among the six databases - the percentage of infants with congenital anomalies ranged from 0.0% in Botswana (0/116 infants) to 13.3% in IMPAACT P1026S (2/15 infants).
Conclusions: Up to 15 million people could be on treatment with DTG in LMICs within the next 5 years, of whom a substantial percentage is likely to be women of child-bearing potential. In many countries with large HIV epidemics, unplanned pregnancies are common and access to antenatal clinic facilities may be limited. Continued pharmacovigilance is essential, but it is reassuring that no clear safety signals have been detected, to date, for pregnant women treated with DTG in terms of birth outcomes or congenital anomalies.
References
-
- World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2nd edn 2016. Available at: www.who.int/hiv/pub/arv/arv-2016/en ( accessed March 2018). - PubMed
-
- AIDSinfo Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV; 2017. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf ( accessed March 2018).
-
- European AIDS Clinical Society Guidelines 2017. Available at: www.eacsociety.org/files/guidelines_8.2-english.pdf ( accessed March 2018).
-
- Walmsley S, Baumgarten A, Berenguer J et al. . Brief report: Dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naïve patients: week 96 and week 144 results from the SINGLE randomised clinical trial. J Acquir Immune Defic Syndr 2015; 70: 515– 519. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources