Bioequivalent pharmacokinetics for generic and originator hepatitis C direct-acting antivirals
- PMID: 29682307
- PMCID: PMC5892672
- DOI: 10.1016/S2055-6640(20)30257-0
Bioequivalent pharmacokinetics for generic and originator hepatitis C direct-acting antivirals
Abstract
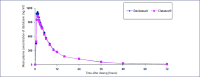
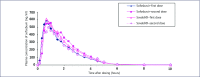
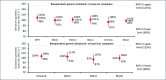
Mass production of low-cost, generic direct-acting antivirals (DAAs) will be required to achieve targets of eliminating hepatitis C (HCV) by 2030. The pharmaceutical companies Gilead and Bristol-Myers Squibb have granted voluntary licences (VLs) to generic companies to mass produce the DAAs sofosbuvir and daclatasvir at low cost. However, generic manufacturers need to demonstrate bioequivalent pharmacokinetics for their DAAs, compared to the originator versions, to fulfil World Health Organization standards for prequalification. The aim of this study was to determine whether generic forms of sofosbuvir and daclatasvir had bioequivalent pharmacokinetics to the originator versions. Generic companies were contacted for results of bioequivalence studies with sofosbuvir and daclatasvir, two of the most widely used DAAs in the developing world. Data on maximum concentration (Cmax) and area under the curve (AUC) were compiled from five generic companies. Pre-specified limits for the 90% confidence intervals were 80-125% of the originator pharmacokinetic concentrations for AUC, and 69-145% for Cmax. The pharmacokinetics of generic sofosbuvir and daclatasvir were shown to be bioequivalent to the originator versions for all five generic companies. This is a crucial step towards securing prequalification of the manufacture of these drugs from these companies. WHO prequalification of bioequivalent generic DAAs could then permit their export to eligible countries for mass-treatment programmes. Mass-treatment with low-cost generic HCV DAAs is the most promising method to achieve the ambitious World Health Organization targets for HCV elimination by 2030.
Keywords: hepatitis C, HCV, direct-acting antivirals, bioequivalence, generics, sofosbuvir, daclatasvir.
Figures
References
-
- Gotham D, Hill A, Barber M et al. Generic treatments for HIV, HBV, HCV, TB could be mass produced for <$90 per patient. 9th IAS Conference on HIV Science. July 2017. Paris, France. Abstract TUAD0104.
-
- Foster GR, Gane E, Asatryan A et al. ENDURANCE-3: safety and efficacy of glecaprevir/pibrentasvir compared to sofosbuvir plus daclatasvir in treatment-naïve HCV genotype 3-infected patients without cirrhosis. J Hepatol 2017; 66: S33.
-
- World Health Organization Global report on access to hepatitis C treatment: focus on overcoming barriers. 2016. Available at: http://apps.who.int/iris/bitstream/10665/250625/1/WHO-HIV-2016.20-eng.pd... ( accessed March 2018).
-
- Doss W. The Egyptian HCV Control Program; 2016. International Viral Hepatitis Elimination (IVHEM) 2017. November 2017. Amsterdam, the Netherlands Available at: http://regist2.virology-education.com/2016/IVHEM/04_Doss.pdf ( accessed March 2018).
-
- World Health Organization Prequalification of medicines by WHO. Available at: www.who.int/mediacentre/factsheets/fs278/en/ ( accessed March 2018).
Publication types
LinkOut - more resources
Full Text Sources
