Kinetics of circulating cell-free DNA for biomedical applications: critical appraisal of the literature
- PMID: 29682327
- PMCID: PMC5905581
- DOI: 10.4155/fsoa-2017-0140
Kinetics of circulating cell-free DNA for biomedical applications: critical appraisal of the literature
Abstract
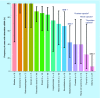
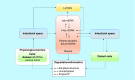
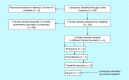
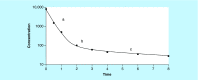
Circulating cell-free DNA is considered as one of the major breakthroughs in the field of innovative diagnosis, used as a liquid biopsy. The kinetic parameters of a biomarker are mandatory to assess its usefulness as a diagnostic tool. Obtaining precise mathematical values for the kinetic parameters (e.g., half-life) is then crucial because it could be used for therapeutic monitoring as a prognostic factor. However, little is known about the intrinsic properties of circulating cell-free DNA, more especially, its kinetic properties within the organism. We summarized the basic principles that may affect the kinetics of circulating cell-free DNA within the organism in the light of biological and clinical evidence. We also meta-analyzed the reported data in the literature and the methodologies that have been used to study the kinetic parameters of human circulating cell-free DNA in vivo.
Keywords: biomarkers; cell-free DNA; half-life; personalized medicine; pharmacokinetics.
Conflict of interest statement
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
