Initiation and Propagation of Vascular Calcification Is Regulated by a Concert of Platelet- and Smooth Muscle Cell-Derived Extracellular Vesicles
- PMID: 29682509
- PMCID: PMC5897433
- DOI: 10.3389/fcvm.2018.00036
Initiation and Propagation of Vascular Calcification Is Regulated by a Concert of Platelet- and Smooth Muscle Cell-Derived Extracellular Vesicles
Abstract
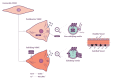
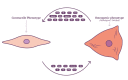
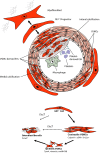
The ageing population continues to suffer from its primary killer, cardiovascular disease (CVD). Despite recent advances in interventional medicinal and surgical therapies towards the end of the 20th century, the epidemic of cardiovascular disease has not been halted. Yet, rather than receding globally, the burden of CVD has risen to become a top cause of morbidity and mortality worldwide. Most CVD arises from thrombotic rupture of an atherosclerotic plaque, the pathologic thickening of coronary and carotid artery segments and subsequent distal ischemia in heart or brain. In fact, one-fifth of deaths are directly attributable to thrombotic rupture of a vulnerable plaque. Atherosclerotic lesion formation is caused by a concert of interactions between circulating leukocytes and platelets, interacting with the endothelial barrier, signalling into the arterial wall by the release of cytokines and extracellular vesicles (EVs). Both platelet- and cell-derived EVs represent a novel mechanism of cellular communication, particularly by the transport and transfer of cargo and by reprogramming of the recipient cell. These interactions result in phenotypic switching of vascular smooth muscle cells (VSMCs) causing migration and proliferation, and subsequent secretion of EVs. Loss of VSMCs attracts perivascular Mesenchymal Stem Cells (MSCs) from the adventitia, which are a source of VSMCs and contribute to repair after vascular injury. However, continuous stress stimuli eventually switch phenotype of cells into osteochondrogenic VSMCs facilitating vascular calcification. Although Virchow's triad is over 100 years old, it is a reality that is accurate today. It can be briefly summarised as changes in the composition of blood (platelet EVs), alterations in the vessel wall (VSMC phenotypic switching, MSC infiltration and EV release) and disruption of blood flow (atherothrombosis). In this paper, we review the latest relevant advances in the identification of extracellular vesicle pathways as well as VSMCs and pericyte/MSC phenotypic switching, underlying vascular calcification.
Keywords: extracellular vesicles; perivascular mesenchymal stem cells; phenotypic switching; platelets; vascular calcification; vascular smooth muscle cells.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources