Dual Affinity Heparin-Based Hydrogels Achieve Pro-Regenerative Immunomodulation and Microvascular Remodeling
- PMID: 29682605
- PMCID: PMC5909722
- DOI: 10.1021/acsbiomaterials.6b00706
Dual Affinity Heparin-Based Hydrogels Achieve Pro-Regenerative Immunomodulation and Microvascular Remodeling
Abstract
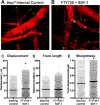
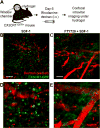
The immune response to biomaterial implants critically regulates functional outcomes such as vascularization, transplant integration/survival, and fibrosis. To create "immunologically smart" materials, the host-material response may be engineered to optimize the recruitment of pro-regenerative leukocyte subsets which mature into corresponding wound-healing macrophages. We have recently identified a unique feature of pro-regenerative Ly6Clow monocytes that is a higher expression of both the bioactive lipid receptor sphingosine-1-phosphate receptor 3 (S1PR3) and the stromal derived factor-1α (SDF-1α) receptor CXCR4. Therefore, we designed a bifunctional hydrogel to harnesses a mechanistic synergy between these signaling axes to enhance the recruitment of endogenous pro-regenerative monocytes. To overcome the challenge of codelivering two physiochemically distinct molecules-a large hydrophilic protein and hydrophobic small molecule-we engineered a dual affinity hydrogel that exploits the growth factor affinity of a heparin derivative (Hep-N) and lipid chaperone activity of albumin. The sphingosine analog FTY720 and SDF-1α are successfully loaded and coreleased from the Hep-N-functionalized PEG-DA hydrogels while maintaining bioactivity. Placement of these hydrogels into a murine partial thickness skin wound demonstrates that corelease of FTY720 and SDF-1α yields superior recruitment of myeloid cells to the implant interface compared to either factor alone. Although in vivo delivery of FTY720 or SDF-1α individually promotes the enhanced recruitment of Ly-6Clow anti-inflammatory monocytes, codelivery enhances the early accumulation and persistence of the differentiated wound healing CD206+ macrophages in the tissue surrounding the gel. Co-delivery similarly promoted the synergistic expansion of vasculature adjacent to the implant, a key step in tissue healing. Taken together, these findings suggest that the combination of chemotactic molecules may provide additional maturation signals to the infiltrating leukocytes to facilitate macrophage transition and vascular network expansion, thus, ultimately, potentiating tissue repair. The coupling of multiple pro-regenerative biological cues provides a foundation for more fine-tuned immunoregenerative modulation to facilitate tissue repair.
Keywords: bioactive lipids; heparin hydrogels; immunoregenerative engineering; stromal derived factor-1α (SDF-1α).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

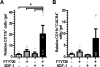
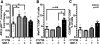


References
-
- Takeda Y, Costa S, Delamarre E, Roncal C, Leite de, Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479(7371):122–6. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
