Determination of minimal steady-state plasma level of diazepam causing seizure threshold elevation in rats
- PMID: 29682729
- PMCID: PMC5934328
- DOI: 10.1111/epi.14069
Determination of minimal steady-state plasma level of diazepam causing seizure threshold elevation in rats
Abstract
Objective: Diazepam, administered by the intravenous, oral, or rectal routes, is widely used for the management of acute seizures. Dosage forms for delivery of diazepam by other routes of administration, including intranasal, intramuscular, and transbuccal, are under investigation. In predicting what dosages are necessary to terminate seizures, the minimal exposure required to confer seizure protection must be known. Here we administered diazepam by continuous intravenous infusion to obtain near-steady-state levels, which allowed an assessment of the minimal levels that elevate seizure threshold.
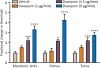
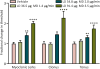
Methods: The thresholds for various behavioral seizure signs (myoclonic jerk, clonus, and tonus) were determined with the timed intravenous pentylenetetrazol seizure threshold test in rats. Diazepam was administered to freely moving animals by continuous intravenous infusion via an indwelling jugular vein cannula. Blood samples for assay of plasma levels of diazepam and metabolites were recovered via an indwelling cannula in the contralateral jugular vein.
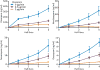
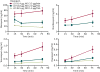
Results: The pharmacokinetic parameters of diazepam following a single 80-μg/kg intravenous bolus injection were determined using a noncompartmental pharmacokinetic approach. The derived parameters Vd , CL, t1/2α (distribution half-life) and t1/2β (terminal half-life) for diazepam were, respectively, 608 mL, 22.1 mL/min, 13.7 minutes, and 76.8 minutes, respectively. Various doses of diazepam were continuously infused without or with an initial loading dose. At the end of the infusions, the thresholds for various behavioral seizure signs were determined. The minimal plasma diazepam concentration associated with threshold elevations was estimated at approximately 70 ng/mL. The active metabolites nordiazepam, oxazepam, and temazepam achieved levels that are expected to make only minor contributions to the threshold elevations.
Significance: Diazepam elevates seizure threshold at steady-state plasma concentrations lower than previously recognized. The minimally effective plasma concentration provides a reference that may be considered when estimating the diazepam exposure required for acute seizure treatment.
Keywords: continuous infusion; diazepam; pharmacokinetics; seizure threshold; time intravenous pentylenetetrazol seizure test.
Wiley Periodicals, Inc. © 2018 International League Against Epilepsy.
Conflict of interest statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. The authors declare no competing financial interests.
Figures

Similar articles
-
Assessment of pharmacokinetics and tolerability of intranasal diazepam relative to rectal gel in healthy adults.Epilepsy Res. 2014 Sep;108(7):1204-11. doi: 10.1016/j.eplepsyres.2014.04.007. Epub 2014 May 13. Epilepsy Res. 2014. PMID: 24934774 Clinical Trial.
-
Intranasal Allopregnanolone Confers Rapid Seizure Protection: Evidence for Direct Nose-to-Brain Delivery.Neurotherapeutics. 2021 Jan;18(1):544-555. doi: 10.1007/s13311-020-00985-5. Epub 2021 Jan 6. Neurotherapeutics. 2021. PMID: 33405197 Free PMC article.
-
Diazepam pharmacokinetics after nasal drop and atomized nasal administration in dogs.J Vet Pharmacol Ther. 2011 Feb;34(1):17-24. doi: 10.1111/j.1365-2885.2010.01186.x. J Vet Pharmacol Ther. 2011. PMID: 21219339 Clinical Trial.
-
Intranasal therapies for acute seizures.Epilepsy Behav. 2015 Aug;49:303-6. doi: 10.1016/j.yebeh.2015.04.027. Epub 2015 May 26. Epilepsy Behav. 2015. PMID: 26022649 Review.
-
Diazepam buccal film for the treatment of acute seizures.Epilepsy Behav. 2019 Dec;101(Pt B):106537. doi: 10.1016/j.yebeh.2019.106537. Epub 2019 Nov 5. Epilepsy Behav. 2019. PMID: 31699662 Review.
Cited by
-
Rescue therapies for seizure clusters: Pharmacology and target of treatments.Epilepsia. 2022 Sep;63 Suppl 1(Suppl 1):S34-S44. doi: 10.1111/epi.17341. Epilepsia. 2022. PMID: 35999174 Free PMC article. Review.
-
Exploring proposed recommendations for immediate-use seizure medication: Treating both cluster and prolonged seizures with diazepam nasal spray.Epilepsia Open. 2025 Aug;10(4):999-1008. doi: 10.1002/epi4.70054. Epub 2025 May 10. Epilepsia Open. 2025. PMID: 40347447 Free PMC article. Review.
-
A randomized, open-label, two-treatment crossover study to evaluate the effect of food on the pharmacokinetics of diazepam nasal spray in healthy adults.Epilepsia. 2023 Feb;64(2):364-373. doi: 10.1111/epi.17459. Epub 2022 Dec 21. Epilepsia. 2023. PMID: 36413125 Free PMC article. Clinical Trial.
-
Pharmacokinetics and safety of VALTOCO (NRL-1; diazepam nasal spray) in patients with epilepsy during seizure (ictal/peri-ictal) and nonseizure (interictal) conditions: A phase 1, open-label study.Epilepsia. 2020 May;61(5):935-943. doi: 10.1111/epi.16506. Epub 2020 Apr 27. Epilepsia. 2020. PMID: 32338380 Free PMC article. Clinical Trial.
-
The unmet need for rapid epileptic seizure termination (REST).Epilepsy Behav Rep. 2020 Nov 25;15:100409. doi: 10.1016/j.ebr.2020.100409. eCollection 2021. Epilepsy Behav Rep. 2020. PMID: 33490947 Free PMC article. Review.
References
-
- Greenfield JL, Sahayak K, Shihabuddin B, Tietz EI, Rosenberg HC. Benzodiazepines (Chapter 50) In: Wyllie E, Gidal BE, Goodkin HP, Loddenkemper T, Sirven J, editors. Wyllie’s Treatment of Epilepsy. Principles and Practice. Sixth. Wolters Kluwer; Philadelphia, PA: 2015. pp. 593–614.
-
- Haut SR, Seinfeld S, Pellock J. Benzodiazepine use in seizure emergencies: A systematic review. Epilepsy Behav. 2016;63:109–117. - PubMed
-
- Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, Bare M, Bleck T, Dodson WE, Garrity L, Jagoda A, Lowenstein D, Pellock J, Riviello J, Sloan E, Treiman DM. Evidence-based guideline: Treatment of convulsive status epilepticus in children and adults: Report of the guideline committee of the American Epilepsy Society. Epilepsy Curr. 2016;16:48–61. - PMC - PubMed
-
- Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta neurologica Scandinavica. 2008;118:69–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

