Rac-GTPases and Rac-GEFs in neutrophil adhesion, migration and recruitment
- PMID: 29682742
- PMCID: PMC6321979
- DOI: 10.1111/eci.12939
Rac-GTPases and Rac-GEFs in neutrophil adhesion, migration and recruitment
Abstract
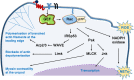
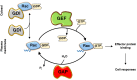
Rac-GTPases and their Rac-GEF activators play important roles in the recruitment and host defence functions of neutrophils. These proteins control the activation of adhesion molecules and the cytoskeletal dynamics that enable the adhesion, migration and tissue recruitment of neutrophils. They also regulate the effector functions that allow neutrophils to kill bacterial and fungal pathogens, and to clear debris. This review focuses on the roles of Rac-GTPases and Rac-GEFs in neutrophil adhesion, migration and recruitment.
Keywords: P-Rex1; Rho-GTPases; Vav; guanine-nucleotide exchange factors; neutrophils; small G proteins.
© The Authors. European Journal of Clinical Investigation published by John Wiley & Sons Ltd on behalf of Stichting European Society for Clinical Investigation Journal Foundation.
Figures




Similar articles
-
Dock2 generates characteristic spatiotemporal patterns of Rac activity to regulate neutrophil polarisation, migration and phagocytosis.Front Immunol. 2023 Jun 13;14:1180886. doi: 10.3389/fimmu.2023.1180886. eCollection 2023. Front Immunol. 2023. PMID: 37383235 Free PMC article.
-
P-Rex1 and Vav1 cooperate in the regulation of formyl-methionyl-leucyl-phenylalanine-dependent neutrophil responses.J Immunol. 2011 Feb 1;186(3):1467-76. doi: 10.4049/jimmunol.1002738. Epub 2010 Dec 22. J Immunol. 2011. PMID: 21178006
-
Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis.Immunity. 2006 Mar;24(3):305-16. doi: 10.1016/j.immuni.2006.02.005. Immunity. 2006. PMID: 16546099
-
Small GTPases and their guanine-nucleotide exchange factors and GTPase-activating proteins in neutrophil recruitment.Curr Opin Hematol. 2016 Jan;23(1):44-54. doi: 10.1097/MOH.0000000000000199. Curr Opin Hematol. 2016. PMID: 26619317 Review.
-
Regulation of neutrophil function by Rac GTPases.Curr Opin Hematol. 2003 Jan;10(1):8-15. doi: 10.1097/00062752-200301000-00003. Curr Opin Hematol. 2003. PMID: 12483106 Review.
Cited by
-
Safety evaluation and modulatory effects on innate immune system of pyrazoline-derived compounds.Naunyn Schmiedebergs Arch Pharmacol. 2025 May;398(5):5677-5691. doi: 10.1007/s00210-024-03653-z. Epub 2024 Nov 27. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39601822
-
Identification of P-Rex1 in the Regulation of Liver Cancer Cell Proliferation and Migration via HGF/c-Met/Akt Pathway.Onco Targets Ther. 2020 Sep 24;13:9481-9495. doi: 10.2147/OTT.S265592. eCollection 2020. Onco Targets Ther. 2020. PMID: 33061433 Free PMC article.
-
β2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function.Front Immunol. 2021 Feb 18;11:619925. doi: 10.3389/fimmu.2020.619925. eCollection 2020. Front Immunol. 2021. PMID: 33679708 Free PMC article. Review.
-
The Rac-GEF Tiam1 controls integrin-dependent neutrophil responses.Front Immunol. 2023 Nov 21;14:1223653. doi: 10.3389/fimmu.2023.1223653. eCollection 2023. Front Immunol. 2023. PMID: 38077328 Free PMC article.
-
Dock2 generates characteristic spatiotemporal patterns of Rac activity to regulate neutrophil polarisation, migration and phagocytosis.Front Immunol. 2023 Jun 13;14:1180886. doi: 10.3389/fimmu.2023.1180886. eCollection 2023. Front Immunol. 2023. PMID: 37383235 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

