The selective mineralocorticoid receptor antagonist eplerenone prevents decompensation of the liver in cirrhosis
- PMID: 29682743
- PMCID: PMC6016674
- DOI: 10.1111/bph.14341
The selective mineralocorticoid receptor antagonist eplerenone prevents decompensation of the liver in cirrhosis
Abstract
Background and purpose: The mineralocorticoid receptor (MR) contributes to fibrosis in various tissues, and MR antagonists, like eplerenone, are used to prevent fibrosis. The role of MR antagonists in hepatic fibrosis and cirrhosis is unknown. Here, we investigated the role of MRs and eplerenone in cirrhosis development.
Experimental approach: Liver fibrosis (5 weeks) and cirrhosis, without (8 weeks) and with ascites (12 weeks), were induced by CCl4 in rats and comprehensively analysed. The effect of eplerenone on the development of cirrhosis with ascites was assessed. MR expression, cellular and subcellular distribution and impact of hypoxia were investigated in vivo and ex vivo. Primary rat hepatocytes and cell lines were used to investigate MR trafficking and transcriptional activity mechanistically.
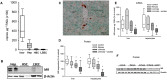
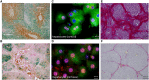
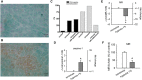
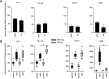
Key results: In cirrhosis with ascites, MR mRNA and protein expressions were reduced in hepatocytes of hypoxic areas. While in normoxic areas MRs were mainly cytosolic, the remaining MRs in hypoxic areas were mainly localized in the nuclei, indicating activation followed by translocation and degradation. Accordingly, eplerenone treatment prevented nuclear MR translocation and the worsening of cirrhosis. Exposing hepatocytes ex vivo to hypoxia induced nuclear MR translocation and enhanced transcriptional MR activity at response elements of the NF-κB pathway.
Conclusions and implications: We showed for the first time that hypoxia leads to a pathogenetic ligand-independent activation of hepatic MRs during cirrhosis resulting in their nuclear translocation and transcriptional activation of the NF-κB pathway. Treatment with eplerenone prevented the worsening of cirrhosis by blocking this ligand-independent activation of the MR.
© 2018 The British Pharmacological Society.
Figures
Similar articles
-
Eplerenone, a mineralocorticoid receptor inhibitor, reduces cirrhosis associated changes of hepatocyte glucose and lipid metabolism.Cell Commun Signal. 2024 Dec 20;22(1):614. doi: 10.1186/s12964-024-01991-2. Cell Commun Signal. 2024. PMID: 39707386 Free PMC article.
-
Beneficial effects of mineralocorticoid receptor blockade in experimental non-alcoholic steatohepatitis.Liver Int. 2015 Sep;35(9):2129-38. doi: 10.1111/liv.12794. Epub 2015 Feb 23. Liver Int. 2015. PMID: 25646700 Free PMC article.
-
Mineralocorticoid receptor (MR) antagonist eplerenone and MR modulator balcinrenone prevent renal extracellular matrix remodeling and inflammation via the MR/proteoglycan/TLR4 pathway.Clin Sci (Lond). 2024 Aug 21;138(16):1025-1038. doi: 10.1042/CS20240302. Clin Sci (Lond). 2024. PMID: 39092535
-
Molecular mechanisms of mineralocorticoid receptor antagonism by eplerenone.Mini Rev Med Chem. 2005 Aug;5(8):709-18. doi: 10.2174/1389557054553811. Mini Rev Med Chem. 2005. PMID: 16101407 Review.
-
Mineralocorticoid Receptor Blockers: Novel Selective Nonsteroidal Mineralocorticoid Receptor Antagonists.Curr Hypertens Rep. 2020 Feb 29;22(3):21. doi: 10.1007/s11906-020-1023-y. Curr Hypertens Rep. 2020. PMID: 32114686 Review.
Cited by
-
Maternal hepatic immunology during pregnancy.Front Immunol. 2023 Jun 30;14:1220323. doi: 10.3389/fimmu.2023.1220323. eCollection 2023. Front Immunol. 2023. PMID: 37457700 Free PMC article. Review.
-
Eplerenone, a mineralocorticoid receptor inhibitor, reduces cirrhosis associated changes of hepatocyte glucose and lipid metabolism.Cell Commun Signal. 2024 Dec 20;22(1):614. doi: 10.1186/s12964-024-01991-2. Cell Commun Signal. 2024. PMID: 39707386 Free PMC article.
-
The expanding class of mineralocorticoid receptor modulators: New ligands for kidney, cardiac, vascular, systemic and behavioral selective actions.Acta Endocrinol (Buchar). 2020 Oct-Dec;16(4):487-496. doi: 10.4183/aeb.2020.487. Acta Endocrinol (Buchar). 2020. PMID: 34084241 Free PMC article. Review.
-
Autophagic degradation of MVBs in LSECs promotes Aldosterone induced-HSCs activation.Hepatol Int. 2024 Feb;18(1):273-288. doi: 10.1007/s12072-023-10559-0. Epub 2023 Jun 18. Hepatol Int. 2024. PMID: 37330971
-
Development of novel liver-targeting glucocorticoid prodrugs.Med Drug Discov. 2024 Feb;21:100172. doi: 10.1016/j.medidd.2023.100172. Epub 2023 Nov 21. Med Drug Discov. 2024. PMID: 38390434 Free PMC article.
References
-
- Aurich H, Koenig S, Schneider C, Walldorf J, Krause P, Fleig WE et al (2005). Functional characterization of serum‐free cultured rat hepatocytes for downstream transplantation applications. Cell Transplant 14: 497–506. - PubMed
-
- Baker ME, Funder JW, Kattoula SR (2013). Evolution of hormone selectivity in glucocorticoid and mineralocorticoid receptors. J Steroid Biochem Mol Biol 137: 57–70. - PubMed
-
- Bataller R, Sancho‐bru P, Ginès P, Brenner DA (2005). Liver fibrogenesis: a new role for the renin–angiotensin system. Antioxid Redox Signal 7: 1346–1355. - PubMed
-
- Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V (2015). Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 63: 1272–1284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

