Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer
- PMID: 29682899
- PMCID: PMC6456815
- DOI: 10.1111/1759-7714.12641
Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer
Abstract
Background: Strict eligibility criteria for patient enrollment in phase III trials raise questions regarding generalization to ineligible patients. We evaluated whether pivotal phase III trials of immune checkpoint blockades (ICBs) represent the overall population of non-small cell lung cancer (NSCLC) patients.
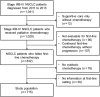
Methods: We reviewed the inclusion and exclusion criteria of three phase III trials (CheckMate057, CheckMate017, and KEYNOTE-010). Stage IIIB or IV NSCLC patients diagnosed from 2011 to 2013 at Seoul National University Hospital (cohort 1) were reviewed. We also analyzed the criteria in 53 patients with NSCLC who were treated with nivolumab or pembrolizumab as routine practice (cohort 2).
Results: Among the 715 patients in cohort 1, 499 (69.9%) were ineligible for the three trials. Reasons for ineligibility included: no prior platinum doublet treatment (23.6%), lack of tissue availability (22.7%), Eastern Cooperative Oncology Group performance status > 1 (14.1%), steroid use (18.2%), active cerebral nervous system metastasis (8.3%), hepatitis B/hepatitis C/human immunodeficiency virus (8.0%), and no measurable lesion (7.3%). EGFR mutations were more common in the ineligible group. In cohort 2, 67.9% of patients were classified as ineligible. Treatment outcomes of ICB in cohort 2 appeared inferior to those in the three pivotal trials, with a response rate of 11.3% and median progression-free survival of 1.67 months.
Conclusion: Only 30% of NSCLC patients were eligible for ICB phase III trials. The actual efficacy in the 70% of ineligible patients is unknown. These findings suggest a huge gap between practice-changing phase III trials and the overall population of NSCLC patients.
Keywords: Clinical trial; eligibility; generalization; immune checkpoint blockade; non-small cell lung cancer.
© 2018 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures


References
-
- Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. - PubMed
-
- National Comprehensive Cancer Network . Non‐Small Cell Lung Cancer (version 6. 2017). 2017. [Cited 9 Feb 2018.] Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
-
- Burstein HJ, Krilov L, Aragon‐Ching JB et al Clinical cancer advances 2017: Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 2017; 35: 1341–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

