Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts
- PMID: 29683202
- PMCID: PMC6038006
- DOI: 10.1111/cei.13130
Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts
Abstract
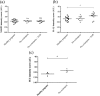
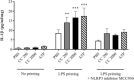
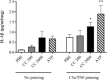
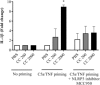
Pre-eclampsia is associated with increased levels of cholesterol and uric acid and an inflamed placenta expressing danger-sensing pattern recognition receptors (PRRs). Crystalline cholesterol and uric acid activate the PRR Nod-like receptor protein (NLRP)3 inflammasome to release interleukin (IL)-1β and result in vigorous inflammation. We aimed to characterize crystal-induced NLRP3 activation in placental inflammation and examine its role in pre-eclampsia. We confirmed that serum total cholesterol and uric acid were elevated in pre-eclamptic compared to healthy pregnancies and correlated positively to high sensitivity C-reactive protein (hsCRP) and the pre-eclampsia marker soluble fms-like tyrosine kinase-1 (sFlt-1). The NLRP3 inflammasome pathway components (NLRP3, caspase-1, IL-1β) and priming factors [complement component 5a (C5a) and terminal complement complex (TCC)] were co-expressed by the syncytiotrophoblast layer which covers the placental surface and interacts with maternal blood. The expression of IL-1β and TCC was increased significantly and C5a-positive regions in the syncytiotrophoblast layer appeared more frequent in pre-eclamptic compared to normal pregnancies. In-vitro activation of placental explants and trophoblasts confirmed NLRP3 inflammasome pathway functionality by complement-primed crystal-induced release of IL-1β. This study confirms crystal-induced NLRP3 inflammasome activation located at the syncytiotrophoblast layer as a mechanism of placental inflammation and suggests contribution of enhanced NLRP3 activation to the harmful placental inflammation in pre-eclampsia.
Keywords: NLRP3; cholesterol; inflammation; placenta; pre-eclampsia.
© 2018 British Society for Immunology.
Figures




References
-
- Watts DH, Krohn MA, Wener MH, Eschenbach DA. C‐reactive protein in normal pregnancy. Obstet Gynecol 1991; 77:176–80. - PubMed
-
- Redman CW, Sargent IL. Preeclampsia and the systemic inflammatory response. Semin Nephrol 2004; 24:565–70. - PubMed
-
- Kalinderis M, Papanikolaou A, Kalinderi K et al Elevated serum levels of interleukin‐6, interleukin‐1beta and human chorionic gonadotropin in pre‐eclampsia. Am J Reprod Immunol 2011; 66:468–75. - PubMed
-
- Ernst GD, de Jonge LL, Hofman A et al C‐reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the generation R study. Am J Obstet Gynecol 2011; 205:132.e1–12. - PubMed
-
- Duley L. The global impact of pre‐eclampsia and eclampsia. Semin Perinatol 2009; 33:130–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

