A novel tricarbonylmethane agent (CMC2.24) reduces human pancreatic tumor growth in mice by targeting Ras
- PMID: 29683208
- PMCID: PMC6461215
- DOI: 10.1002/mc.22830
A novel tricarbonylmethane agent (CMC2.24) reduces human pancreatic tumor growth in mice by targeting Ras
Abstract
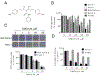
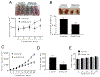
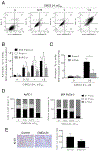
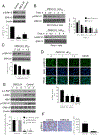
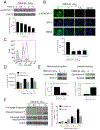
Pancreatic Cancer (PC) is a deadly disease in need of new therapeutic options. We recently developed a novel tricarbonylmethane agent (CMC2.24) as a therapeutic agent for PC, and evaluated its efficacy in preclinical models of PC. CMC2.24 inhibited the growth of various human PC cell lines in a concentration and time-dependent manner. Normal human pancreatic epithelial cells were resistant to CMC2.24, indicating selectivity. CMC2.24 reduced the growth of subcutaneous and orthotopic PC xenografts in mice by up to 65% (P < 0.02), and the growth of a human patient-derived tumor xenograft by 47.5% (P < 0.03 vs vehicle control). Mechanistically, CMC2.24 inhibited the Ras-RAF-MEK-ERK pathway. Based on Ras Pull-Down Assays, CMC2.24 inhibited Ras-GTP, the active form of Ras, in MIA PaCa-2 cells and in pancreatic acinar explants isolated from Kras mutant mice, by 90.3% and 89.1%, respectively (P < 0.01, for both). The inhibition of active Ras led to an inhibition of c-RAF, MEK, and ERK phosphorylation by 93%, 91%, and 87%, respectively (P < 0.02, for all) in PC xenografts. Furthermore, c-RAF overexpression partially rescued MIA PaCa-2 cells from the cell growth inhibition by CMC2.24. In addition, downstream of ERK, CMC2.24 inhibited STAT3 phosphorylation levels at the serine 727 residue, enhanced the levels of superoxide anion in mitochondria, and induced intrinsic apoptosis as shown by the release of cytochrome c from the mitochondria to the cytosol and the further cleavage of caspase 9 in PC cells. In conclusion, CMC2.24, a potential Ras inhibitor, is an efficacious agent for PC treatment in preclinical models, deserving further evaluation.
Keywords: CMC2.24; ERK; Kras; Ras; curcumin; pancreatic cancer.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures
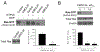
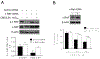
Similar articles
-
A novel Ras inhibitor (MDC-1016) reduces human pancreatic tumor growth in mice.Neoplasia. 2013 Oct;15(10):1184-95. doi: 10.1593/neo.131368. Neoplasia. 2013. PMID: 24204197 Free PMC article.
-
Embelin suppresses growth of human pancreatic cancer xenografts, and pancreatic cancer cells isolated from KrasG12D mice by inhibiting Akt and Sonic hedgehog pathways.PLoS One. 2014 Apr 2;9(4):e92161. doi: 10.1371/journal.pone.0092161. eCollection 2014. PLoS One. 2014. PMID: 24694877 Free PMC article.
-
Rasfonin, a novel 2-pyrone derivative, induces ras-mutated Panc-1 pancreatic tumor cell death in nude mice.Cell Death Dis. 2014 May 22;5(5):e1241. doi: 10.1038/cddis.2014.213. Cell Death Dis. 2014. PMID: 24853419 Free PMC article.
-
PAR-4 as a possible new target for pancreatic cancer therapy.Expert Opin Ther Targets. 2010 Jun;14(6):611-20. doi: 10.1517/14728222.2010.487066. Expert Opin Ther Targets. 2010. PMID: 20426700 Free PMC article. Review.
-
Inhibition of pancreatic cancer cell growth.Cell Mol Life Sci. 2007 Oct;64(19-20):2512-21. doi: 10.1007/s00018-007-7072-4. Cell Mol Life Sci. 2007. PMID: 17676272 Free PMC article. Review.
Cited by
-
EGCG sensitizes chemotherapeutic-induced cytotoxicity by targeting the ERK pathway in multiple cancer cell lines.Arch Biochem Biophys. 2020 Oct 15;692:108546. doi: 10.1016/j.abb.2020.108546. Epub 2020 Aug 18. Arch Biochem Biophys. 2020. PMID: 32818507 Free PMC article.
-
Novel Chemically Modified Curcumin (CMC) Derivatives Inhibit Tyrosinase Activity and Melanin Synthesis in B16F10 Mouse Melanoma Cells.Biomolecules. 2021 Apr 30;11(5):674. doi: 10.3390/biom11050674. Biomolecules. 2021. PMID: 33946371 Free PMC article.
-
Phospho-valproic acid (MDC-1112) reduces pancreatic cancer growth in patient-derived tumor xenografts and KPC mice: enhanced efficacy when combined with gemcitabine.Carcinogenesis. 2020 Jul 14;41(7):927-939. doi: 10.1093/carcin/bgz170. Carcinogenesis. 2020. PMID: 31584613 Free PMC article.
-
A Novel Modified-Curcumin Promotes Resolvin-Like Activity and Reduces Bone Loss in Diabetes-Induced Experimental Periodontitis.J Inflamm Res. 2021 Oct 16;14:5337-5347. doi: 10.2147/JIR.S330157. eCollection 2021. J Inflamm Res. 2021. PMID: 34703272 Free PMC article.
-
Hyaluronic acid nanoparticle-encapsulated microRNA-125b repolarizes tumor-associated macrophages in pancreatic cancer.Nanomedicine (Lond). 2021 Oct;16(25):2291-2303. doi: 10.2217/nnm-2021-0080. Epub 2021 Sep 28. Nanomedicine (Lond). 2021. PMID: 34579548 Free PMC article.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–2921. - PubMed
-
- Kleeff J, Korc M, Apte M et al. Pancreatic cancer. Nature reviews Disease primers 2016;2:16022. - PubMed
-
- Hruban RH, van Mansfeld AD, Offerhaus GJ et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. The American journal of pathology 1993;143(2):545–554. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

