Axonal neurofilaments exhibit frequent and complex folding behaviors
- PMID: 29683261
- PMCID: PMC6019213
- DOI: 10.1002/cm.21448
Axonal neurofilaments exhibit frequent and complex folding behaviors
Abstract
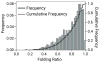
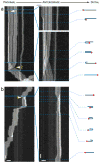
Neurofilaments are flexible cytoskeletal polymers that are capable of folding and unfolding between their bouts of bidirectional movement along axons. Here we present a detailed characterization of this behavior in cultured neurons using kymograph analysis with approximately 30 ms temporal resolution. We analyzed 781 filaments ranging from 0.6-42 µm in length. We observed complex behaviors including pinch folds, hairpin folds, orientation changes (flips), and occasional severing and annealing events. On average, the filaments spent approximately 40% of their time in some sort of folded configuration. A small proportion of filaments (4%) moved while folded, but most (96%) moved in an outstretched configuration. Collectively, our observations suggest that motors may interact with neurofilaments at multiple points along their length, but preferentially at their ends. In addition, the prevalence of neurofilament folding and the tendency of neurofilaments to straighten out when they move, suggest that an important function of the movement of these polymers in axons may be to maintain them in an outstretched and longitudinally co-aligned configuration. Thus, neurofilament movement may function as much to organize these polymers as to move them, and this could explain why they spend so much time engaged in apparently unproductive bidirectional movement.
Keywords: annealing; axonal transport; kymograph; neurofilament; severing.
© 2018 Wiley Periodicals, Inc.
Figures









References
-
- Beck R, Deek J, Jones JB, Safinya CR. Gel-expanded to gel-condensed transition in neurofilament networks revealed by direct force measurements. Nat Mater. 2010a;9(1):40–6. - PubMed
-
- Beck R, Deek J, Choi MC, Ikawa T, Watanabe O, Frey E, Pincus P, Safinya CR. Unconventional salt trend from soft to stiff in single neurofilament biopolymers. Langmuir. 2010b;26(24):18595–9. - PubMed
-
- Brown A. Slow axonal transport: stop and go traffic in the axon. Nat Rev Mol Cell Biol. 2000;1:153–56. - PubMed
-
- Brown A. Slow axonal transport. In: Caplan M, editor. Reference Module in the Biomedical Sciences. Elsevier; 2014.
-
- Brown A. Axonal transport. In: Pfaff DW, Volkow ND, editors. Neuroscience in the 21st century: from basic to clinical. New York: Springer Publishing; 2015. pp. 333–79.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

