Regulation of hemolysin in uropathogenic Escherichia coli fine-tunes killing of human macrophages
- PMID: 29683762
- PMCID: PMC5989160
- DOI: 10.1080/21505594.2018.1465786
Regulation of hemolysin in uropathogenic Escherichia coli fine-tunes killing of human macrophages
Abstract
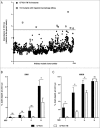
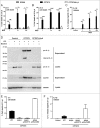
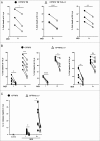
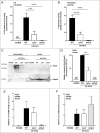
Uropathogenic E. coli (UPEC) causes the majority of urinary tract infections (UTIs), which are a major global public health concern. UPEC uses numerous mechanisms to subvert the innate immune system, including targeting macrophage functions. We recently showed that some UPEC strains rapidly kill human macrophages via an NLRP3-independent pathway, and also trigger NLRP3-dependent IL-1β processing. In this study, we used random transposon mutagenesis in the reference strain CFT073 to identify UPEC genes that mediate human macrophage cell death. Our approach revealed that the hemolysin A (HlyA) toxin is essential for triggering both cell death and NLRP3 inflammasome-mediated IL-1β release in human macrophages. Random transposon mutagenesis also identified the cof gene, which encodes a poorly characterized phosphatase, as a novel hemolysin regulator; a CFT073 mutant deleted for the cof gene secreted significantly reduced levels of HlyA, had diminished hemolytic activity, and was impaired in its capacity to trigger human macrophage cell death and IL-1β release. Together, our findings reveal that Cof fine-tunes production of hemolysin, an important determinant of both UPEC-mediated inflammasome activation and human macrophage cell death.
Keywords: UPEC; cell death; cof; gene regulation; inflammasome; macrophage; urinary tract infection; α-hemolysin.
Figures
Similar articles
-
Variation in hemolysin A expression between uropathogenic Escherichia coli isolates determines NLRP3-dependent vs. -independent macrophage cell death and host colonization.FASEB J. 2019 Jun;33(6):7437-7450. doi: 10.1096/fj.201802100R. Epub 2019 Mar 14. FASEB J. 2019. PMID: 30869997
-
Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection.Proc Natl Acad Sci U S A. 2015 Feb 24;112(8):E871-80. doi: 10.1073/pnas.1500374112. Epub 2015 Feb 9. Proc Natl Acad Sci U S A. 2015. PMID: 25675528 Free PMC article.
-
Activation of the NLRP3 Inflammasome Pathway by Uropathogenic Escherichia coli Is Virulence Factor-Dependent and Influences Colonization of Bladder Epithelial Cells.Front Cell Infect Microbiol. 2018 Mar 14;8:81. doi: 10.3389/fcimb.2018.00081. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29662840 Free PMC article.
-
Hemolysin of uropathogenic Escherichia coli: A cloak or a dagger?Biochim Biophys Acta. 2016 Mar;1858(3):538-45. doi: 10.1016/j.bbamem.2015.08.015. Epub 2015 Aug 20. Biochim Biophys Acta. 2016. PMID: 26299820 Review.
-
'Omic' Approaches to Study Uropathogenic Escherichia coli Virulence.Trends Microbiol. 2017 Sep;25(9):729-740. doi: 10.1016/j.tim.2017.04.006. Epub 2017 May 24. Trends Microbiol. 2017. PMID: 28550944 Review.
Cited by
-
"Omics" Technologies - What Have They Told Us About Uropathogenic Escherichia coli Fitness and Virulence During Urinary Tract Infection?Front Cell Infect Microbiol. 2022 Feb 14;12:824039. doi: 10.3389/fcimb.2022.824039. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35237532 Free PMC article. Review.
-
Reaching the End of the Line: Urinary Tract Infections.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.bai-0014-2019. doi: 10.1128/microbiolspec.BAI-0014-2019. Microbiol Spectr. 2019. PMID: 31172909 Free PMC article. Review.
-
Genomic insight into the high-risk hypervirulent multidrug resistant enteroaggregative-hemorrhagic Escherichia coli ST648/*a194 (serotype O8:H4) isolated from a 3-year-old patient with bloodstream infection in Uganda, sub-Saharan Africa.Gene Rep. 2025 Jun;39:102198. doi: 10.1016/j.genrep.2025.102198. Epub 2025 Mar 14. Gene Rep. 2025. PMID: 40235845
-
α-Hemolysin of uropathogenic E. coli regulates NLRP3 inflammasome activation and mitochondrial dysfunction in THP-1 macrophages.Sci Rep. 2020 Jul 28;10(1):12653. doi: 10.1038/s41598-020-69501-1. Sci Rep. 2020. PMID: 32724079 Free PMC article.
-
In Silico Prediction and Design of Uropathogenic Escherichia coli Alpha-Hemolysin Generate a Soluble and Hemolytic Recombinant Toxin.Microorganisms. 2022 Jan 14;10(1):172. doi: 10.3390/microorganisms10010172. Microorganisms. 2022. PMID: 35056621 Free PMC article.
References
-
- Ragnarsdottir B, Lutay N, Gronberg-Hernandez J, et al.. Genetics of innate immunity and UTI susceptibility. Nat Rev Urol. 2011;8:449–68. - PubMed
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. - PubMed
-
- Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–60. - PubMed
-
- Aboderin OA, Abdu AR, Odetoyin BW, et al.. Antimicrobial resistance in Escherichia coli strains from urinary tract infections. J Natl Med Assoc. 2009;101:1268–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
