Circulating inflammatory monocytes contribute to impaired influenza vaccine responses in HIV-infected participants
- PMID: 29683844
- PMCID: PMC8728748
- DOI: 10.1097/QAD.0000000000001821
Circulating inflammatory monocytes contribute to impaired influenza vaccine responses in HIV-infected participants
Abstract
Objective: Antibody responses are often impaired in old age and in HIV-positive (HIV+) infection despite virologic control with antiretroviral therapy but innate immunologic determinants are not well understood.
Design: Monocytes and natural killer cells were examined for relationships to age, HIV infection and influenza vaccine responses.
Methods: Virologically suppressed HIV+ (n = 139) and HIV-negative (HIV-) (n = 137) participants classified by age as young (18-39 years), middle-aged (40-59 years) and old (≥60 years) were evaluated preinfluenza and postinfluenza vaccination.
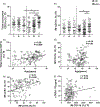
Results: Prevaccination frequencies of inflammatory monocytes were highest in old HIV+ and HIV-, with old HIV+ exhibiting higher frequency of integrin CD11b on inflammatory monocytes that was correlated with age, expression of C-C chemokine receptor-2 (CCR2) and plasma soluble tumor necrosis factor receptor-1 (sTNFR1), with inverse correlation with postvaccination influenza H1N1 antibody titers. Higher frequencies of CD11b+ inflammatory monocytes (CD11b(hi), >48.4%) compared with low frequencies of CD11b+ inflammatory monocytes (<15.8%) was associated with higher prevaccination frequencies of total and inflammatory monocytes and higher CCR2 MFI, higher plasma sTNFR1 and CXCL-10 with higher lipopolysaccharide stimulated expression of TNFα and IL-6, concomitant with lower postvaccination influenza antibody titers. In HIV+ CD11b(hi) expressers, the depletion of inflammatory monocytes from peripheral blood mononuclear cells resulted in enhanced antigen-specific CD4+ T-cell proliferation. Immature CD56(hi) natural killer cells were lower in young HIV+ compared with young HIV- participants.
Conclusion: Perturbations of innate immunity and inflammation signified by high CD11b on inflammatory monocytes are exacerbated with aging in HIV+ and negatively impact immune function involved in Ab response to influenza vaccination.
Conflict of interest statement
Conflicts of interest
There are no conflicts of interest.
Figures

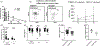
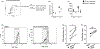
Similar articles
-
Impact of aging and HIV infection on serologic response to seasonal influenza vaccination.AIDS. 2018 Jun 1;32(9):1085-1094. doi: 10.1097/QAD.0000000000001774. AIDS. 2018. PMID: 29424779 Free PMC article.
-
Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation.PLoS One. 2013 Nov 13;8(11):e79816. doi: 10.1371/journal.pone.0079816. eCollection 2013. PLoS One. 2013. PMID: 24236161 Free PMC article.
-
HIV infection Worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine.J Infect Dis. 2015 Jun 15;211(12):1959-68. doi: 10.1093/infdis/jiu840. Epub 2015 Jan 2. J Infect Dis. 2015. PMID: 25556252 Free PMC article.
-
Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy.J Immunol. 2011 Jun 1;186(11):6173-81. doi: 10.4049/jimmunol.1100264. Epub 2011 Apr 29. J Immunol. 2011. PMID: 21531891 Free PMC article.
-
Response to 2009 pandemic and seasonal influenza vaccines co-administered to HIV-infected and HIV-uninfected former drug users living in a rehabilitation community in Italy.Vaccine. 2011 Nov 15;29(49):9209-13. doi: 10.1016/j.vaccine.2011.09.103. Epub 2011 Oct 3. Vaccine. 2011. PMID: 21974995
Cited by
-
The Suppressive Attitude of Inflammatory Monocytes in Antiviral Antibody Responses.Viral Immunol. 2020 May;33(4):327-333. doi: 10.1089/vim.2019.0132. Epub 2020 Feb 6. Viral Immunol. 2020. PMID: 32027238 Free PMC article. Review.
-
Impaired B Cell Function in Mice Lacking Perforin-2.Front Immunol. 2020 Feb 27;11:328. doi: 10.3389/fimmu.2020.00328. eCollection 2020. Front Immunol. 2020. PMID: 32180773 Free PMC article.
-
Single Cell Profiling Reveals PTEN Overexpression in Influenza-Specific B cells in Aging HIV-infected individuals on Anti-retroviral Therapy.Sci Rep. 2019 Feb 21;9(1):2482. doi: 10.1038/s41598-019-38906-y. Sci Rep. 2019. PMID: 30792481 Free PMC article.
-
Systems analysis of immune responses to attenuated P. falciparum malaria sporozoite vaccination reveals excessive inflammatory signatures correlating with impaired immunity.PLoS Pathog. 2022 Feb 2;18(2):e1010282. doi: 10.1371/journal.ppat.1010282. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35108339 Free PMC article. Clinical Trial.
-
Immune response to the recombinant herpes zoster vaccine in people living with HIV over 50 years of age compared to non-HIV age-/gender-matched controls (SHINGR'HIV): a multicenter, international, non-randomized clinical trial study protocol.BMC Infect Dis. 2024 Mar 19;24(1):329. doi: 10.1186/s12879-024-09192-5. BMC Infect Dis. 2024. PMID: 38504173 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC). Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices –— United States, 2013–2014. MMWR Recomm Rep 2013; 62 (RR07):1–43. - PubMed
-
- Kaplan-Lewis E, Aberg JA, Lee M. Aging with HIV in the ART era. Semin Diagn Pathol 2017; 34:384–397. - PubMed
-
- Appay V, Kelleher AD. Immune activation and immune aging in HIV infection. Curr Opin HIV AIDS 2016; 11:242–249. - PubMed
-
- Fulop T, Herbein G, Cossarizza A, Witkowski JM, Frost E, Dupuis G, et al. Cellular senescence, immunosenescence and HIV. Interdiscip Top Gerontol Geriatr 2017; 42:28–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

