Seroconversion on preexposure prophylaxis: a case report with segmental hair analysis for timed adherence determination
- PMID: 29683856
- PMCID: PMC6140333
- DOI: 10.1097/QAD.0000000000001825
Seroconversion on preexposure prophylaxis: a case report with segmental hair analysis for timed adherence determination
Abstract
Objective: We describe the third case report of seroconversion with multidrug resistant (MDR)-HIV despite pre-exposure prophylaxis (PrEP) with emtricitabine (FTC) and tenofovir (TFV) disoproxil (TDF).
Design: Case report.
Methods: PrEP adherence was assessed via self-report, pharmacy records, and measuring TFV/FTC levels with liquid-chromatography/tandem-mass-spectrometry in plasma and hair. Segmental hair analysis was performed to assess PrEP adherence over prior months. Genotypic resistance was assessed.
Results: A 34 year-old white MSM started daily FTC/TDF in February 2016 after being provided 11 refills. In March 2017, he developed fevers, chills, myalgias and was assessed, but no HIV test was sent. In April 2017, an antigen/antibody HIV test was reactive (day 0). On day 2, HIV-1 RNA was 27 316 copies/ml, genotyping revealed M184V, K70T, K65R, and K103N mutations, plasma TFV and FTC concentrations were consistent with recent dosing. To evaluate adherence over preceding months, a hair sample was collected at day 27 and segmental analysis of TFV/FTC levels performed in one-centimeter segments from the scalp. Hair drug levels (0.0434-0.0520 ng TFV/mg hair) were commensurate with consistently high PrEP adherence over the prior 3 months.
Conclusions: This study employs segmental analysis of PrEP drug levels in hair for the first time to assess adherence over preceding months in the setting of an HIV seroconversion on PrEP. High adherence over the period of likely acquisition makes MDR-HIV infection the most likely scenario in this case. Adequate adherence assessment when examining PrEP failures and ensuring best practices in PrEP prescribing and follow-up are important.
Conflict of interest statement
Conflicts of interest
All authors have no conflicts of interest to declare. Written informed consent to publish in all formats was obtained from the patient described in this study.
Previously presented in abstract form at the 25th Annual Conference on Retroviruses and Opportunistic Infections (CROI): Thaden JT, Gandhi M, Kuncze K,
Figures
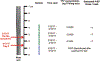
Comment in
-
Seroconversion on preexposure prophylaxis.AIDS. 2018 Jun 1;32(9):1199-1200. doi: 10.1097/QAD.0000000000001826. AIDS. 2018. PMID: 29746319 No abstract available.
Similar articles
-
Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States.Clin Infect Dis. 2018 Jan 6;66(2):213-219. doi: 10.1093/cid/cix755. Clin Infect Dis. 2018. PMID: 29020194 Free PMC article.
-
Similar tenofovir hair concentrations in men and women after directly observed dosing of tenofovir disoproxil fumarate/emtricitabine: implications for preexposure prophylaxis adherence monitoring.AIDS. 2018 Sep 24;32(15):2189-2194. doi: 10.1097/QAD.0000000000001935. AIDS. 2018. PMID: 30212404 Free PMC article.
-
Tenofovir and emtricitabine concentrations in hair are comparable between individuals on tenofovir disoproxil fumarate versus tenofovir alafenamide-based ART.Drug Test Anal. 2021 Jul;13(7):1354-1370. doi: 10.1002/dta.3033. Epub 2021 Apr 13. Drug Test Anal. 2021. PMID: 33742745 Free PMC article.
-
Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis?Curr Opin HIV AIDS. 2016 Jan;11(1):49-55. doi: 10.1097/COH.0000000000000209. Curr Opin HIV AIDS. 2016. PMID: 26633640 Free PMC article. Review.
-
Nondaily preexposure prophylaxis for HIV prevention.Curr Opin HIV AIDS. 2016 Jan;11(1):94-101. doi: 10.1097/COH.0000000000000213. Curr Opin HIV AIDS. 2016. PMID: 26633641 Free PMC article. Review.
Cited by
-
Consensus statement on human immunodeficiency virus pre-exposure prophylaxis in China.Chin Med J (Engl). 2020 Dec 5;133(23):2840-2846. doi: 10.1097/CM9.0000000000001181. Chin Med J (Engl). 2020. PMID: 33273333 Free PMC article. No abstract available.
-
First Case of HIV Seroconversion With Integrase Resistance Mutations on Long-Acting Cabotegravir for Prevention in Routine Care.Open Forum Infect Dis. 2024 Aug 26;11(9):ofae468. doi: 10.1093/ofid/ofae468. eCollection 2024 Sep. Open Forum Infect Dis. 2024. PMID: 39229286 Free PMC article.
-
Importance of early identification of PrEP breakthrough infections in a generalized HIV epidemic: a case report from a PrEP demonstration project in South Africa.BMC Infect Dis. 2020 Jul 22;20(1):532. doi: 10.1186/s12879-020-05255-5. BMC Infect Dis. 2020. PMID: 32698772 Free PMC article.
-
Tenofovir, emtricitabine, lamivudine and dolutegravir concentrations in plasma and urine following drug intake cessation in a randomized controlled directly observed pharmacokinetic trial to aid point-of-care testing.J Antimicrob Chemother. 2024 Jul 1;79(7):1597-1605. doi: 10.1093/jac/dkae147. J Antimicrob Chemother. 2024. PMID: 38758205 Free PMC article. Clinical Trial.
-
Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report.Lancet HIV. 2018 Nov 29:S2352-3018(18)30288-1. doi: 10.1016/S2352-3018(18)30288-1. Online ahead of print. Lancet HIV. 2018. PMID: 30503324 Free PMC article.
References
-
- Knox DC, Anderson PL, Harrigan PR, Tan DH. Multidrug-resistant HIV-1 infection despite preexposure prophylaxis. N Engl J Med 2017; 376:501–502. - PubMed
-
- Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.−2006. AIDS 2010; 24:1203–1212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

