Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates
- PMID: 29684038
- PMCID: PMC5912768
- DOI: 10.1371/journal.pone.0196080
Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates
Abstract
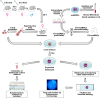
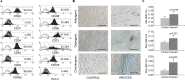
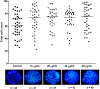
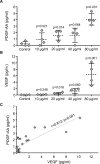
Endometrial Mesenchymal Stromal Cells (endMSCs) are multipotent cells with immunomodulatory and pro-regenerative activity which is mainly mediated by a paracrine effect. The exosomes released by MSCs have become a promising therapeutic tool for the treatment of immune-mediated diseases. More specifically, extracellular vesicles derived from endMSCs (EV-endMSCs) have demonstrated a cardioprotective effect through the release of anti-apoptotic and pro-angiogenic factors. Here we hypothesize that EV-endMSCs may be used as a co-adjuvant to improve in vitro fertilization outcomes and embryo quality. Firstly, endMSCs and EV-endMSCs were isolated and phenotypically characterized for in vitro assays. Then, in vitro studies were performed on murine embryos co-cultured with EV-endMSCs at different concentrations. Our results firstly demonstrated a significant increase on the total blastomere count of expanded murine blastocysts. Moreover, EV-endMSCs triggered the release of pro-angiogenic molecules from embryos demonstrating an EV-endMSCs concentration-dependent increase of VEGF and PDGF-AA. The release of VEGF and PDGF-AA by the embryos may indicate that the beneficial effect of EV-endMSCs could be mediating not only an increase in the blastocyst's total cell number, but also may promote endometrial angiogenesis, vascularization, differentiation and tissue remodeling. In summary, these results could be relevant for assisted reproduction being the first report describing the beneficial effect of human EV-endMSCs on embryo development.
Conflict of interest statement
Figures

References
-
- Khoury M, Alcayaga-Miranda F, Illanes SE, Figueroa FE. The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front Immunol. 2014;5: 205 doi: 10.3389/fimmu.2014.00205 - DOI - PMC - PubMed
-
- Uder C, Brückner S, Winkler S, Tautenhahn H-M, Christ B. Mammalian MSC from selected species: Features and applications. Cytometry A. 2018;93: 32–49. doi: 10.1002/cyto.a.23239 - DOI - PubMed
-
- Burke J, Hunter M, Kolhe R, Isales C, Hamrick M, Fulzele S. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin Transl Med. 2016;5: 27 doi: 10.1186/s40169-016-0112-7 - DOI - PMC - PubMed
-
- Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32: 2818–2823. doi: 10.1002/stem.1818 - DOI - PubMed
-
- Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23: 812–823. doi: 10.1038/mt.2015.44 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

