Development and validation of brain target controlled infusion of propofol in mice
- PMID: 29684039
- PMCID: PMC5912730
- DOI: 10.1371/journal.pone.0194949
Development and validation of brain target controlled infusion of propofol in mice
Abstract
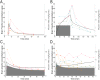
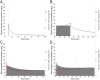
Mechanisms through which anesthetics disrupt neuronal activity are incompletely understood. In order to study anesthetic mechanisms in the intact brain, tight control over anesthetic pharmacology in a genetically and neurophysiologically accessible animal model is essential. Here, we developed a pharmacokinetic model that quantitatively describes propofol distribution into and elimination out of the brain. To develop the model, we used jugular venous catheters to infuse propofol in mice and measured propofol concentration in serial timed brain and blood samples using high performance liquid chromatography (HPLC). We then used adaptive fitting procedures to find parameters of a three compartment pharmacokinetic model such that all measurements collected in the blood and in the brain across different infusion schemes are fit by a single model. The purpose of the model was to develop target controlled infusion (TCI) capable of maintaining constant brain propofol concentration at the desired level. We validated the model for two different targeted concentrations in independent cohorts of experiments not used for model fitting. The predictions made by the model were unbiased, and the measured brain concentration was indistinguishable from the targeted concentration. We also verified that at the targeted concentration, state of anesthesia evidenced by slowing of the electroencephalogram and behavioral unresponsiveness was attained. Thus, we developed a useful tool for performing experiments necessitating use of anesthetics and for the investigation of mechanisms of action of propofol in mice.
Conflict of interest statement
Figures

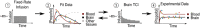

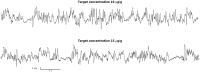
Similar articles
-
Evaluation and optimisation of propofol pharmacokinetic parameters in cats for target-controlled infusion.Vet Rec. 2016 May 14;178(20):503. doi: 10.1136/vr.103560. Epub 2016 Apr 4. Vet Rec. 2016. PMID: 27044652
-
[Accuracy of target-controlled infusion (TCI) with 2 different propofol formulations].Anaesthesist. 2004 Oct;53(10):937-43. doi: 10.1007/s00101-004-0753-6. Anaesthesist. 2004. PMID: 15372176 German.
-
The performance of compartmental and physiologically based recirculatory pharmacokinetic models for propofol: a comparison using bolus, continuous, and target-controlled infusion data.Anesth Analg. 2010 Aug;111(2):368-79. doi: 10.1213/ANE.0b013e3181bdcf5b. Epub 2009 Oct 27. Anesth Analg. 2010. PMID: 19861357
-
Are there still limitations for the use of target-controlled infusion in children?Curr Opin Anaesthesiol. 2010 Jun;23(3):356-62. doi: 10.1097/ACO.0b013e32833938db. Curr Opin Anaesthesiol. 2010. PMID: 20386438 Review.
-
Pharmacokinetic models for propofol--defining and illuminating the devil in the detail.Br J Anaesth. 2009 Jul;103(1):26-37. doi: 10.1093/bja/aep143. Epub 2009 Jun 10. Br J Anaesth. 2009. PMID: 19520702 Review.
Cited by
-
Modeling propofol-induced cardiotoxicity in the isolated-perfused newborn mouse heart.Physiol Rep. 2022 Aug;10(15):e15402. doi: 10.14814/phy2.15402. Physiol Rep. 2022. PMID: 35923108 Free PMC article.
-
Explaining anaesthetic hysteresis with effect-site equilibration.Br J Anaesth. 2021 Jan;126(1):265-278. doi: 10.1016/j.bja.2020.09.022. Epub 2020 Oct 17. Br J Anaesth. 2021. PMID: 33081972 Free PMC article.
-
Robust alternative to the righting reflex to assess arousal in rodents.Sci Rep. 2020 Nov 20;10(1):20280. doi: 10.1038/s41598-020-77162-3. Sci Rep. 2020. PMID: 33219247 Free PMC article.
-
Novel Approach for Characterizing Propofol Binding Affinities to Serum Albumins from Different Species.ACS Omega. 2020 Sep 30;5(40):25543-25551. doi: 10.1021/acsomega.0c01295. eCollection 2020 Oct 13. ACS Omega. 2020. PMID: 33073080 Free PMC article.
-
Coherence of Visual-Evoked Gamma Oscillations Is Disrupted by Propofol but Preserved Under Equipotent Doses of Isoflurane.Front Syst Neurosci. 2019 May 8;13:19. doi: 10.3389/fnsys.2019.00019. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31139058 Free PMC article.
References
-
- Brown EN, Lydic R, Schiff ND. General Anesthesia, Sleep, and Coma. N Engl J Med. 2010; 2638–2650. doi: 10.1056/NEJMra0808281 - DOI - PMC - PubMed
-
- Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34: 601–628. doi: 10.1146/annurev-neuro-060909-153200 - DOI - PMC - PubMed
-
- Hemmings HC. Molecular targets of general anesthetics in the nervous system. Hudetz A, Pearce R, editors. Totowa, NJ: Humana Press; 2010.
-
- Joiner WJ, Friedman EB, Hung HT, Koh K, Sowcik M, Sehgal A, et al. Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness. PLoS Genet. 2013;9: 1–12. doi: 10.1371/journal.pgen.1003605 - DOI - PMC - PubMed
-
- Friedman EB, Sun Y, Moore JT, Hung H-T, Meng QC, Perera P, et al. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One. 2010;5: e11903 doi: 10.1371/journal.pone.0011903 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

