Ovostatin 2 knockdown significantly inhibits the growth, migration, and tumorigenicity of cutaneous malignant melanoma cells
- PMID: 29684087
- PMCID: PMC5912766
- DOI: 10.1371/journal.pone.0195610
Ovostatin 2 knockdown significantly inhibits the growth, migration, and tumorigenicity of cutaneous malignant melanoma cells
Abstract
Background: We previously identified ovostatin 2 (OVOS2) as a new candidate gene for cutaneous malignant melanoma (CMM) in a Chinese population. In this study, we aimed to investigate the exact role of OVOS2 in cell proliferation, invasion, and tumorigenesis of melanoma A375 cells.
Methods: The downregulation of OVOS2 expression was performed using lentiviral vectors with specific shRNA. The effects of OVOS2 expression on cell proliferation, cell cycle, cell migration, cell invasion, and potential of tumorigenesis were further investigated.
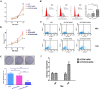
Results: The downregulation of OVOS2 significantly suppressed the proliferation of A375 cells and led to a G2/M phase block. The transwell cell migration assay showed that the reduced expression of OVOS2 also significantly inhibited the transmigration of A375 cells. The western blot results showed downregulated expression of p-FAK, p-AKT, and p-ERK. This was accompanied by the upregulated epithelial phenotypes E-cadherin and β-catenin, and downregulated expression of mesenchymal phenotype N-cadherin after OVOS2 knockdown. The transplantation tumor experiment in BALB/C nude mouse showed that after an observation period of 32 days, the growth speed and weight of the transplanted tumors were significantly suppressed in the BALB/c nude mice subcutaneously injected with OVOS2 knocked-down A375 cells.
Conclusion: The inhibition of OVOS2 had significant suppressive effects on the proliferation, motility, and migration capabilities of A375 cells, suggesting a crucial promotive role of OVOS2 in the pathogenesis and progression of CMM. The involved mechanisms are at least partly associated with the overactivation of FAK/MAPK/ERK and FAK/PI3K/AKT signals.
Conflict of interest statement
Figures



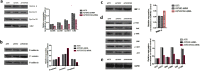

Similar articles
-
Expression analysis of ovostatin 2 reveals its involvement in proliferation, invasion and angiogenesis of cutaneous malignant melanoma.J Dermatol. 2013 Nov;40(11):901-10. doi: 10.1111/1346-8138.12294. Epub 2013 Sep 23. J Dermatol. 2013. PMID: 24112097
-
FGFR3 promotes the growth and malignancy of melanoma by influencing EMT and the phosphorylation of ERK, AKT, and EGFR.BMC Cancer. 2019 Oct 16;19(1):963. doi: 10.1186/s12885-019-6161-8. BMC Cancer. 2019. PMID: 31619201 Free PMC article.
-
Overexpressed VEPH1 inhibits epithelial-mesenchymal transition, invasion, and migration of human cutaneous melanoma cells through inactivating the TGF-β signaling pathway.Cell Cycle. 2019 Nov;18(21):2860-2875. doi: 10.1080/15384101.2019.1638191. Epub 2019 Sep 4. Cell Cycle. 2019. Retraction in: Cell Cycle. 2022 Jul;21(13):1435. doi: 10.1080/15384101.2022.2066278. PMID: 31599708 Free PMC article. Retracted.
-
Viewing malignant melanoma cells as macrophage-tumor hybrids.Cell Adh Migr. 2007 Jan-Mar;1(1):2-6. Epub 2007 Jan 15. Cell Adh Migr. 2007. PMID: 19262091 Free PMC article. Review.
-
Effects of infections on the pathogenesis of cancer.Indian J Med Res. 2021 Apr;153(4):431-445. doi: 10.4103/ijmr.IJMR_339_19. Indian J Med Res. 2021. PMID: 34380789 Free PMC article. Review.
Cited by
-
A complex of novel protease inhibitor, ovostatin homolog, with its cognate proteases in immature mice uterine luminal fluid.Sci Rep. 2019 Mar 21;9(1):4973. doi: 10.1038/s41598-019-41426-4. Sci Rep. 2019. PMID: 30899053 Free PMC article.
-
Elucidation of anti-human melanoma and anti-aging mechanisms of compounds from green seaweed Caulerpa racemosa.Sci Rep. 2024 Nov 11;14(1):27534. doi: 10.1038/s41598-024-78464-6. Sci Rep. 2024. PMID: 39528552 Free PMC article.
-
Decreased expression of SCARA5 predicts a poor prognosis in melanoma using bioinformatics analysis.Front Oncol. 2023 Mar 24;13:1015358. doi: 10.3389/fonc.2023.1015358. eCollection 2023. Front Oncol. 2023. PMID: 37035142 Free PMC article.
References
-
- National Cancer Institute. Cancer Stat Facts: Melanoma of the Skin. SEER.2017 [cited 2017 26 May]. Available from: http://seer.cancer.gov/statfacts/html/melan.html.
-
- Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC Med. 2012;10:23 doi: 10.1186/1741-7015-10-23 - DOI - PMC - PubMed
-
- Welinder C, Pawlowski K, Szasz AM, Yakovleva M, Sugihara Y, Malm J, et al. Correlation of histopathologic characteristics to protein expression and function in malignant melanoma. PLoS One. 2017;12:e0176167 doi: 10.1371/journal.pone.0176167 - DOI - PMC - PubMed
-
- Marzuka A, Huang L, Theodosakis N, Bosenberg M. Melanoma Treatments: Advances and Mechanisms. J Cell Physiol. 2015;230:2626–2633. doi: 10.1002/jcp.25019 - DOI - PubMed
-
- Vockley JG, Niederhuber JE. Diagnosis and treatment of cancer using genomics. BMJ. 2015;350:h1832 doi: 10.1136/bmj.h1832 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

