Maternal vitamin D supplementation during pregnancy
- PMID: 29684104
- PMCID: PMC6003599
- DOI: 10.1093/bmb/ldy010
Maternal vitamin D supplementation during pregnancy
Abstract
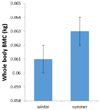
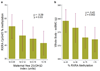
Introduction: Maternal vitamin D status in pregnancy has been linked to many health outcomes in mother and offspring. A wealth of observational studies have reported on both obstetric outcomes and complications, including pre-eclampsia, gestational diabetes, mode and timing of delivery. Many foetal and childhood outcomes are also linked to vitamin D status, including measures of foetal size, body composition and skeletal mineralization, in addition to later childhood outcomes, such as asthma.
Sources of data: Synthesis of systematic and narrative reviews.
Areas of agreement and controversy: The findings are generally inconsistent in most areas, and, at present, there is a lack of data from high-quality intervention studies to confirm a causal role for vitamin D in these outcomes. In most areas, the evidence tends towards maternal vitamin D being of overall benefit, but often does not reach statistical significance in meta-analyses.
Growing points and areas timely for developing research: The most conclusive evidence is in the role of maternal vitamin D supplementation in the prevention of neonatal hypocalcaemia; as a consequence the UK department of health recommends that pregnant women take 400 IU vitamin D daily. High-quality randomized placebo-controlled trials, such as the UK-based MAVIDOS trial, will inform the potential efficacy and safety of vitamin D supplementation in pregnancy across a variety of outcomes.
Conflict of interest statement
EMC and RM have no disclosures. NH has no disclosures directly related to this work, and has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare and Internis Pharma; CC has no disclosures directly related to this work, and has received consultancy, lecture fees and honoraria from AMGEN, GSK, Alliance for Better Bone Health, MSD, Eli Lilly, Pfizer, Novartis, Servier, Medtronic and Roche.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 17702/VAC_/Versus Arthritis/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- 21231/VAC_/Versus Arthritis/United Kingdom
- 21231/ARC_/Arthritis Research UK/United Kingdom
- 201222/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- 17702/ARC_/Arthritis Research UK/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- BB/P028179/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- HTA/10/33/04/DH_/Department of Health/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- 10/33/04/DH_/Department of Health/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

