Myeloid Cell Responses to Contraction-induced Injury Differ in Muscles of Young and Old Mice
- PMID: 29684112
- PMCID: PMC6230214
- DOI: 10.1093/gerona/gly086
Myeloid Cell Responses to Contraction-induced Injury Differ in Muscles of Young and Old Mice
Abstract
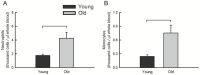
Myeloid cells play a critical role in regulating muscle degeneration and regeneration. Thus, alterations with aging in the myeloid cell response to muscle damage may affect the progression of the injury in old animals. We hypothesized that neutrophil levels remain elevated and that macrophage accumulation is reduced or delayed in injured muscles of old compared with young animals. Muscles of young and old mice were injured with lengthening contractions and analyzed 2 or 5 days later. Regardless of age, neutrophil (Gr-1+) and macrophage (CD68+) content increased dramatically by Day 2. Between 2 and 5 days, macrophages increased further, whereas neutrophils declined to a level that in old muscles was not different from uninjured controls. M2 macrophages (CD163+) also increased between 2 and 5 days, reaching higher levels in muscles of old mice than in young mice. Although no evidence of persisting neutrophils or reduced M2 accumulation in old muscle was found, total macrophage accumulation was lower in old mice. Furthermore, messenger RNA levels showed age-related changes in macrophage-associated genes that may indicate alterations in myeloid cell function. Overall, differences between muscles of old and young mice in the inflammatory response through the early stages of injury may contribute to defects in muscle regeneration.
Figures





Similar articles
-
Neutrophil accumulation following passive stretches contributes to adaptations that reduce contraction-induced skeletal muscle injury in mice.J Appl Physiol (1985). 2008 Apr;104(4):1109-15. doi: 10.1152/japplphysiol.00850.2007. Epub 2008 Feb 14. J Appl Physiol (1985). 2008. PMID: 18276901
-
Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice.J Physiol. 2005 Feb 1;562(Pt 3):899-913. doi: 10.1113/jphysiol.2004.073965. Epub 2004 Nov 18. J Physiol. 2005. PMID: 15550464 Free PMC article.
-
Treatment with selectin blocking antibodies after lengthening contractions of mouse muscle blunts neutrophil accumulation but does not reduce damage.Physiol Rep. 2016 Jan;4(1):e12667. doi: 10.14814/phy2.12667. Physiol Rep. 2016. PMID: 26733249 Free PMC article.
-
Regulatory interactions between muscle and the immune system during muscle regeneration.Am J Physiol Regul Integr Comp Physiol. 2010 May;298(5):R1173-87. doi: 10.1152/ajpregu.00735.2009. Epub 2010 Mar 10. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20219869 Free PMC article. Review.
-
Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention.Phys Ther. 1993 Dec;73(12):911-21. doi: 10.1093/ptj/73.12.911. Phys Ther. 1993. PMID: 8248299 Review.
Cited by
-
Arginine ingestion inhibits phagocyte invasion in eccentrically contracted rat fast-twitch muscle.J Muscle Res Cell Motil. 2024 Dec;45(4):201-209. doi: 10.1007/s10974-024-09672-w. Epub 2024 Apr 18. J Muscle Res Cell Motil. 2024. PMID: 38635146 Free PMC article.
-
Restoring Mitochondrial Function and Muscle Satellite Cell Signaling: Remedies against Age-Related Sarcopenia.Biomolecules. 2024 Mar 28;14(4):415. doi: 10.3390/biom14040415. Biomolecules. 2024. PMID: 38672432 Free PMC article. Review.
-
Phytoecdysteroids Accelerate Recovery of Skeletal Muscle Function Following in vivo Eccentric Contraction-Induced Injury in Adult and Old Mice.Front Rehabil Sci. 2021 Nov 8;2:757789. doi: 10.3389/fresc.2021.757789. eCollection 2021. Front Rehabil Sci. 2021. PMID: 36188800 Free PMC article.
-
Resolvin D1 supports skeletal myofiber regeneration via actions on myeloid and muscle stem cells.JCI Insight. 2020 Sep 17;5(18):e137713. doi: 10.1172/jci.insight.137713. JCI Insight. 2020. PMID: 32750044 Free PMC article.
-
Macrophage Regulation of Muscle Regrowth From Disuse in Aging.Exerc Sport Sci Rev. 2019 Oct;47(4):246-250. doi: 10.1249/JES.0000000000000201. Exerc Sport Sci Rev. 2019. PMID: 31525165 Free PMC article. Review.
References
-
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi:10.1038/nature03260 - PubMed
-
- Sadeh M. Effects of aging on skeletal muscle regeneration. J Neurol Sci. 1988;87:67–74. doi:10.1016/0022-510X(88)90055-X - PubMed
-
- Ghaly A, Marsh DR. Aging-associated oxidative stress modulates the acute inflammatory response in skeletal muscle after contusion injury. Exp Gerontol. 2010;45:381–388. doi:10.1016/j.exger.2010.03.004 - PubMed
-
- Zhou Y, Lovell D, Bethea M, et al. . Age-dependent changes cooperatively impact skeletal muscle regeneration after compartment syndrome injury. Am J Pathol. 2014;184:2225–2236. doi:10.1016/j.ajpath.2014.03.018 - PubMed
-
- McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18:355–357. doi:10.1096/fj.03-0395fje - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

