Multi-modal characterization of vasculature and nanoparticle accumulation in five tumor xenograft models
- PMID: 29684498
- PMCID: PMC5972071
- DOI: 10.1016/j.jconrel.2018.04.026
Multi-modal characterization of vasculature and nanoparticle accumulation in five tumor xenograft models
Abstract
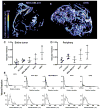
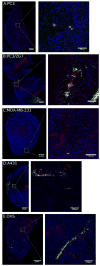
Preclinical research has demonstrated that nanoparticles and macromolecules can accumulate in solid tumors due to the enhanced permeability and retention effect. However, drug loaded nanoparticles often fail to show increased efficacy in clinical trials. A better understanding of how tumor heterogeneity affects nanoparticle accumulation could help elucidate this discrepancy and help in patient selection for nanomedicine therapy. Here we studied five human tumor models with varying morphology and evaluated the accumulation of 100 nm polystyrene nanoparticles. Each tumor model was characterized in vivo using micro-computed tomography, contrast-enhanced ultrasound and diffusion-weighted and dynamic contrast-enhanced magnetic resonance imaging. Ex vivo, the tumors were sectioned for both fluorescence microscopy and histology. Nanoparticle uptake and distribution in the tumors were generally heterogeneous. Density of functional blood vessels measured by fluorescence microscopy correlated significantly (p = 0.0056) with nanoparticle accumulation and interestingly, inflow of microbubbles measured with ultrasound also showed a moderate but significant (p = 0.041) correlation with nanoparticle accumulation indicating that both amount of vessels and vessel morphology and perfusion predict nanoparticle accumulation. This indicates that blood vessel characterization using contrast-enhanced ultrasound imaging or other methods could be valuable for patient stratification for treatment with nanomedicines.
Keywords: MRI; Microscopy; Nanoparticles; Tumor characterization; Tumor vasculature; Ultrasound; microCT.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures








References
-
- Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. - PubMed
-
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J Controlled Release. 2015;200:138–157. - PubMed
-
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1
-
- Ekdawi SN, Jaffray DA, Allen C. Nanomedicine and tumor heterogeneity: Concept and complex reality. Nano Today. 2016;11:402–414.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

