High salt intake enhances swim stress-induced PVN vasopressin cell activation and active stress coping
- PMID: 29684712
- PMCID: PMC6269109
- DOI: 10.1016/j.psyneuen.2018.04.003
High salt intake enhances swim stress-induced PVN vasopressin cell activation and active stress coping
Abstract
Purpose: Stress contributes to many psychiatric disorders; however, responsivity to stressors can vary depending on previous or current stress exposure. Relatively innocuous heterotypic (differing in type) stressors can summate to result in exaggerated neuronal and behavioral responses. Here we investigated the ability of prior high dietary sodium chloride (salt) intake, a dehydrating osmotic stressor, to enhance neuronal and behavioral responses of mice to an acute psychogenic swim stress (SS). Further, we evaluated the contribution of the osmo-regulatory stress-related neuropeptide arginine vasopressin (VP) in the hypothalamic paraventricular nucleus (PVN), one of only a few brain regions that synthesize VP. The purpose of this study was to determine the impact of high dietary salt intake on responsivity to heterotypic stress and the potential contribution of VPergic-mediated neuronal activity on high salt-induced stress modulation, thereby providing insight into how dietary (homeostatic) and environmental (psychogenic) stressors might interact to facilitate psychiatric disorder vulnerability.
Approach: Salt loading (SL) with 4% saline for 7 days was used to dehydrate and osmotically stress mice prior to exposure to an acute SS. Fluid intake and hematological measurements were taken to quantify osmotic dehydration, and serum corticosterone levels were measured to index stress axis activation. Immunohistochemistry (IHC) was used to stain for the immediate early gene product c-Fos to quantify effects of SL on SS-induced activation of neurons in the PVN and extended amygdala - brain regions that are synaptically connected and implicated in responding to osmotic stress and in modulation of SS behavior, respectively. Lastly, the role of VPergic PVN neurons and VP type 1 receptor (V1R) activity in the amygdala in mediating effects of SL on SS behavior was evaluated by quantifying c-Fos activation of VPergic PVN neurons and, in functional experiments, by nano-injecting the V1R selective antagonist dGly[Phaa1,d-tyr(et), Lys, Arg]-VP bilaterally into the amygdala prior to the SS.
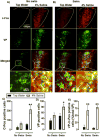
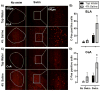
Findings: SL increased serum osmolality (P < 0.01), which positively correlated with time spent mobile during, and time spent grooming after a SS (P < 0.01, P < 0.01), and SL increased serum corticosterone levels (P < 0.01). SL alone increased c-Fos immunoreactivity among PVN neurons (P = .02), including VP positive neurons (P < 0.01). SL increased SS-induced c-Fos activation of PVN neurons as well (P < 0.01). In addition, SL and SS each increased the total number of PVN neurons that were immunoreactive for VP (P < 0.01). An enhancing effect of SL and SS was observed on c-Fos positive cell counts in the central (P = .02) and basolateral (P < 0.01) nuclei of the amygdala and bilateral nano-injections of V1R antagonist into the amygdala reduced time spent mobile both in salt loaded and control mice during SS (P < 0.05, P < 0.05).
Summary: Taken together, these data indicate that neuronal and behavioral responsivity to an acute psychogenic stressor is potentiated by prior exposure to high salt intake. This synergistic effect was associated with activation of PVN VP neurons and depended, in part, on activity of V1 receptors in the amygdala. Findings provide novel insight into neural mechanisms whereby prior exposure to a homeostatic stressor such as osmotic dehydration by excessive salt intake increases responsivity to a perceived stress. These experiments show that high dietary salt can influence stress responsivity and raise the possibility that excessive salt intake could be a contributing factor in the development of stress-related psychiatric disorders.
Keywords: Arginine vasopressin; Dehydration; Hyperosmolality; Paraventricular nucleus; Psychiatric; Salt diet; Stress coping.
Published by Elsevier Ltd.
Conflict of interest statement
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Neuroinflammation Contributes to High Salt Intake-Augmented Neuronal Activation and Active Coping Responses to Acute Stress.Int J Neuropsychopharmacol. 2019 Feb 1;22(2):137-142. doi: 10.1093/ijnp/pyy099. Int J Neuropsychopharmacol. 2019. PMID: 30535261 Free PMC article.
-
Time-dependent sensitization of corticotropin-releasing hormone, arginine vasopressin and c-fos immunoreactivity within the mouse brain in response to tumor necrosis factor-alpha.Neuroscience. 2001;106(1):137-48. doi: 10.1016/s0306-4522(01)00276-7. Neuroscience. 2001. PMID: 11564424
-
Vasopressin-containing neurons of the hypothalamic parvocellular paraventricular nucleus of the jerboa: plasticity related to immobilization stress.Neuroendocrinology. 2006;84(6):396-404. doi: 10.1159/000100509. Epub 2007 Mar 13. Neuroendocrinology. 2006. PMID: 17384516
-
Regulation of pituitary ACTH secretion during chronic stress.Front Neuroendocrinol. 1994 Dec;15(4):321-50. doi: 10.1006/frne.1994.1013. Front Neuroendocrinol. 1994. PMID: 7895891 Review.
-
Modulation of cardiorespiratory function mediated by the paraventricular nucleus.Respir Physiol Neurobiol. 2010 Nov 30;174(1-2):55-64. doi: 10.1016/j.resp.2010.08.001. Epub 2010 Aug 11. Respir Physiol Neurobiol. 2010. PMID: 20708107 Free PMC article. Review.
Cited by
-
Habitual salt preference worsens blood pressure in hospitalized hypertensive patients with omicron infection under epidemic-related stress.BMC Public Health. 2024 Jan 9;24(1):134. doi: 10.1186/s12889-023-17633-0. BMC Public Health. 2024. PMID: 38195459 Free PMC article.
-
Neuroinflammation Contributes to High Salt Intake-Augmented Neuronal Activation and Active Coping Responses to Acute Stress.Int J Neuropsychopharmacol. 2019 Feb 1;22(2):137-142. doi: 10.1093/ijnp/pyy099. Int J Neuropsychopharmacol. 2019. PMID: 30535261 Free PMC article.
-
Effects of diluted seawater in drinking water on physiological responses, feeding, drinking patterns, and water balance in crossbred dairy goats.Vet World. 2024 Oct;17(10):2398-2406. doi: 10.14202/vetworld.2024.2398-2406. Epub 2024 Oct 31. Vet World. 2024. PMID: 39619940 Free PMC article.
-
Glutamate Spillover Dynamically Strengthens Gabaergic Synaptic Inhibition of the Hypothalamic Paraventricular Nucleus.J Neurosci. 2024 Feb 14;44(7):e1851222023. doi: 10.1523/JNEUROSCI.1851-22.2023. J Neurosci. 2024. PMID: 38154957 Free PMC article.
-
Stress and sex-dependent effects on conditioned inhibition of fear.Learn Mem. 2022 Sep 2;29(9):246-255. doi: 10.1101/lm.053508.121. Print 2022 Sep. Learn Mem. 2022. PMID: 36206391 Free PMC article.
References
-
- Amaya F, Tanaka M, Hayashi S, Tanaka Y, Ibata Y. Hypothalamo-pituitary-adrenal axis sensitization after chronic salt loading. Neuroendocrinology. 2001;73(3):185–193. - PubMed
-
- American Heart Association. Life style and risk reduction for high blood pressure. http://www.heart.org/idc/groups/heart-public/@wcm/@hcm/documents/downloa... (Accessed 11 November 2017)
-
- Bankir L, de Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol. 1985;249(6 Pt 2):R643–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

