The body electric 2.0: recent advances in developmental bioelectricity for regenerative and synthetic bioengineering
- PMID: 29684787
- PMCID: PMC10464502
- DOI: 10.1016/j.copbio.2018.03.008
The body electric 2.0: recent advances in developmental bioelectricity for regenerative and synthetic bioengineering
Abstract
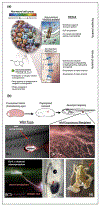
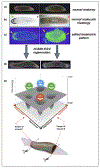
Breakthroughs in biomedicine and synthetic bioengineering require predictive, rational control over anatomical structure and function. Recent successes in manipulating cellular and molecular hardware have not been matched by progress in understanding the patterning software implemented during embryogenesis and regeneration. A fundamental capability gap is driving desired changes in growth and form to address birth defects and traumatic injury. Here we review new tools, results, and conceptual advances in an exciting emerging field: endogenous non-neural bioelectric signaling, which enables cellular collectives to make global decisions and implement large-scale pattern homeostasis. Spatially distributed electric circuits regulate gene expression, organ morphogenesis, and body-wide axial patterning. Developmental bioelectricity facilitates the interface to organ-level modular control points that direct patterning in vivo. Cracking the bioelectric code will enable transformative progress in bioengineering and regenerative medicine.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer.Cell. 2021 Apr 15;184(8):1971-1989. doi: 10.1016/j.cell.2021.02.034. Epub 2021 Apr 6. Cell. 2021. PMID: 33826908 Review.
-
Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form.Annu Rev Biomed Eng. 2017 Jun 21;19:353-387. doi: 10.1146/annurev-bioeng-071114-040647. Annu Rev Biomed Eng. 2017. PMID: 28633567 Free PMC article. Review.
-
The bioelectric code: An ancient computational medium for dynamic control of growth and form.Biosystems. 2018 Feb;164:76-93. doi: 10.1016/j.biosystems.2017.08.009. Epub 2017 Sep 2. Biosystems. 2018. PMID: 28855098 Free PMC article. Review.
-
Modeling somatic computation with non-neural bioelectric networks.Sci Rep. 2019 Dec 9;9(1):18612. doi: 10.1038/s41598-019-54859-8. Sci Rep. 2019. PMID: 31819119 Free PMC article.
-
Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs.Integr Biol (Camb). 2015 Dec;7(12):1487-517. doi: 10.1039/c5ib00221d. Epub 2015 Nov 16. Integr Biol (Camb). 2015. PMID: 26571046 Free PMC article. Review.
Cited by
-
Endogenous Bioelectrics in Development, Cancer, and Regeneration: Drugs and Bioelectronic Devices as Electroceuticals for Regenerative Medicine.iScience. 2019 Dec 20;22:519-533. doi: 10.1016/j.isci.2019.11.023. Epub 2019 Nov 25. iScience. 2019. PMID: 31837520 Free PMC article. Review.
-
Future medicine: from molecular pathways to the collective intelligence of the body.Trends Mol Med. 2023 Sep;29(9):687-710. doi: 10.1016/j.molmed.2023.06.007. Epub 2023 Jul 20. Trends Mol Med. 2023. PMID: 37481382 Free PMC article. Review.
-
Insights into regeneration tool box: An animal model approach.Dev Biol. 2019 Sep 15;453(2):111-129. doi: 10.1016/j.ydbio.2019.04.006. Epub 2019 Apr 13. Dev Biol. 2019. PMID: 30986388 Free PMC article. Review.
-
Assessment of Enrichment of Human Mesenchymal Stem Cells Based on Plasma and Mitochondrial Membrane Potentials.Bioelectricity. 2020 Mar 1;2(1):21-32. doi: 10.1089/bioe.2019.0024. Epub 2020 Mar 18. Bioelectricity. 2020. PMID: 32292894 Free PMC article.
-
Toward Decoding Bioelectric Events in Xenopus Embryogenesis: New Methodology for Tracking Interplay Between Calcium and Resting Potentials In Vivo.J Mol Biol. 2020 Jan 17;432(2):605-620. doi: 10.1016/j.jmb.2019.10.029. Epub 2019 Nov 9. J Mol Biol. 2020. PMID: 31711960 Free PMC article.
References
-
- Varenne F, Chaigneau P, Petitot J, Doursat R: Programming the emergence in morphogenetically architected complex systems. Acta Biotheor 2015, 63:295–308. - PubMed
-
- Pitcairn E, McLaughlin KA: Bioelectric signaling coordinates patterning decisions during embryogenesis. Trends Dev Biol 2016, 9:1–9.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

