The Role of Promyelocytic Leukemia Protein in Steatosis-Associated Hepatic Tumors Related to Chronic Hepatitis B virus Infection
- PMID: 29684791
- PMCID: PMC6050444
- DOI: 10.1016/j.tranon.2018.03.013
The Role of Promyelocytic Leukemia Protein in Steatosis-Associated Hepatic Tumors Related to Chronic Hepatitis B virus Infection
Abstract
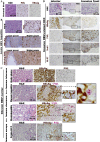
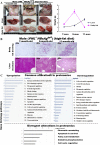
The persistence of hepatitis B surface antigen (HBsAg) is a risk factor for the development of steatosis-associated tumors in chronic hepatitis B virus (HBV) infection, yet little is known about the metabolic link with this factor. We correlated HBV-related pathogenesis in genetically engineered mice and human carriers with metabolic proteomics and lipogenic gene expression profiles. The immunohistochemistry showed that the promyelocytic leukemia protein (PML, a tumor suppressor involved in genome maintenance and fatty acid oxidation), being inversely influenced by the dynamic HBsAg levels from acute phase to seroclearance, appeared as a lipo-metabolic switch linking HBsAg-induced steatosis (lipogenesis) to HBsAg-lost fat-burning hepatocarcinogenesis (lipolysis). Knockdown of PML in HBsAg-transgenic mice predisposed to obesity and drove early steatosis-specific liver tumorigenesis. Proteome analysis revealed that the signaling pathways corresponding to energy metabolism and its regulators were frequently altered by suppression or depletion of PML in the HBsAg-transgenic mice, mainly including oxidative phosphorylation and fatty acid metabolism. Expression profiling further identified upregulation of stearoyl-CoA desaturase 1 (Scd1) and epigenetic methylation of NDUFA13 in the mitochondrial respiratory chain and the cell cycle inhibitor CDKN1c in concordance to the increased severity of lipodystrophy and neoplasia in the livers of HBsAg-transgenic mice with PML insufficiency. The defect in lipolysis in PML-deficient HBsAg-transgenic mice made the HBsAg-induced adipose-like liver tumors vulnerable to synthetic lethality from toxic saturated fat accumulation with a Scd1 inhibitor. Our findings provide mechanistic insights into the evolution of steatosis-associated hepatic tumors driven by reciprocal interactions of HBsAg and PML, and a potential utility of lipid metabolic reprogramming as a treatment target.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures









Similar articles
-
Promyelocytic leukaemia protein links DNA damage response and repair to hepatitis B virus-related hepatocarcinogenesis.J Pathol. 2013 Aug;230(4):377-87. doi: 10.1002/path.4195. J Pathol. 2013. PMID: 23620081
-
Dual oncogenic and tumor suppressor roles of the promyelocytic leukemia gene in hepatocarcinogenesis associated with hepatitis B virus surface antigen.Oncotarget. 2016 May 10;7(19):28393-407. doi: 10.18632/oncotarget.8613. Oncotarget. 2016. PMID: 27058621 Free PMC article.
-
Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection?Int J Obes (Lond). 2007 May;31(5):871-5. doi: 10.1038/sj.ijo.0803479. Epub 2006 Oct 17. Int J Obes (Lond). 2007. PMID: 17047638 Clinical Trial.
-
Stearoyl-CoA desaturase as a new drug target for obesity treatment.Obes Rev. 2005 May;6(2):169-74. doi: 10.1111/j.1467-789X.2005.00177.x. Obes Rev. 2005. PMID: 15836467 Review.
-
Natural history of chronic hepatitis B virus infection: an immunopathological study.J Gastroenterol Hepatol. 1997 Oct;12(9-10):S218-22. doi: 10.1111/j.1440-1746.1997.tb00503.x. J Gastroenterol Hepatol. 1997. PMID: 9407340 Review.
Cited by
-
Cannabinoid receptor 1 knockout alleviates hepatic steatosis by downregulating perilipin 2.Lab Invest. 2020 Mar;100(3):454-465. doi: 10.1038/s41374-019-0327-5. Epub 2019 Sep 30. Lab Invest. 2020. PMID: 31570772 Free PMC article.
-
Angiotensin II promotes ovarian cancer spheroid formation and metastasis by upregulation of lipid desaturation and suppression of endoplasmic reticulum stress.J Exp Clin Cancer Res. 2019 Mar 7;38(1):116. doi: 10.1186/s13046-019-1127-x. J Exp Clin Cancer Res. 2019. PMID: 30845964 Free PMC article.
-
Metabolic Dysfunction-Associated Fatty Liver Disease and Chronic Viral Hepatitis: The Interlink.Pathogens. 2024 Jan 10;13(1):68. doi: 10.3390/pathogens13010068. Pathogens. 2024. PMID: 38251375 Free PMC article. Review.
-
TRIM proteins in hepatocellular carcinoma.J Biomed Sci. 2022 Sep 13;29(1):69. doi: 10.1186/s12929-022-00854-7. J Biomed Sci. 2022. PMID: 36100865 Free PMC article. Review.
-
Cholestasis impairs hepatic lipid storage via AMPK and CREB signaling in hepatitis B virus surface protein transgenic mice.Lab Invest. 2020 Nov;100(11):1411-1424. doi: 10.1038/s41374-020-0457-9. Epub 2020 Jul 1. Lab Invest. 2020. PMID: 32612285 Free PMC article.
References
-
- Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int. 2016;36:1239–1251. - PubMed
-
- Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013;33:147–156. - PubMed
-
- Karagozian R, Derdák Z, Baffy G. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. 2014;63:607–617. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

