Maternal caffeine intake during pregnancy and childhood growth and overweight: results from a large Norwegian prospective observational cohort study
- PMID: 29685923
- PMCID: PMC5914784
- DOI: 10.1136/bmjopen-2017-018895
Maternal caffeine intake during pregnancy and childhood growth and overweight: results from a large Norwegian prospective observational cohort study
Abstract
Objectives: To study the association between maternal caffeine intake during pregnancy and the child's weight gain and overweight risk up to 8 years.
Design: Prospective nationwide pregnancy cohort.
Setting: The Norwegian Mother and Child Cohort Study.
Participants: A total of 50 943 mothers recruited from 2002 to 2008 and their children, after singleton pregnancies, with information about average caffeine intake assessed at mid-pregnancy.
Outcome measure: Child's body size information at 11 age points from 6 weeks to 8 years. We defined excess growth in infancy as a WHO weight gain z-score of >0.67 from birth to age 1 year, and overweight according to the International Obesity Task Force. We used a growth model to assess individual growth trajectories.
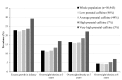
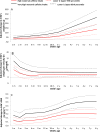
Results: Compared with pregnant women with low caffeine intake (<50 mg/day, 46%), women with average (50-199 mg/day, 44%), high (≥200-299 mg/day, 7%) and very high (≥300 mg/day, 3%) caffeine intakes had an increased risk of their child experiencing excess growth in infancy, after adjustment for confounders (OR=1.15, 95% CI 1.09 to 1.22, OR=1.30, 95% CI 1.16 to 1.45, OR=1.66, 95% CI 1.42 to 1.93, respectively). In utero exposure to any caffeine was associated with higher risk of overweight at age 3 years and 5 years, while the association persisted at 8 years, only for very high exposures. Any caffeine intake was associated with increased body mass index from infancy to childhood. Children prenatally exposed to caffeine intake >200 mg/day had consistently higher weight. Very high caffeine exposures were associated with higher weight gain velocity from infancy to age 8 years.
Conclusion: Any caffeine consumption during pregnancy is associated with a higher risk of excess infant growth and of childhood overweight, mainly at preschool ages. Maternal caffeine intake may modify the overall weight growth trajectory of the child from birth to 8 years. This study adds supporting evidence for the current advice to reduce caffeine intake during pregnancy.
Keywords: epidemiology; preventive medicine; public health; social medicine.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Association of maternal caffeine intake during pregnancy with low birth weight, childhood overweight, and obesity: a meta-analysis of cohort studies.Int J Obes (Lond). 2021 Feb;45(2):279-287. doi: 10.1038/s41366-020-0617-4. Epub 2020 Jun 9. Int J Obes (Lond). 2021. PMID: 32518355 Review.
-
Maternal vitamin D intake and BMI during pregnancy in relation to child's growth and weight status from birth to 8 years: a large national cohort study.BMJ Open. 2021 Oct 1;11(10):e048980. doi: 10.1136/bmjopen-2021-048980. BMJ Open. 2021. PMID: 34598984 Free PMC article.
-
Fish Intake in Pregnancy and Child Growth: A Pooled Analysis of 15 European and US Birth Cohorts.JAMA Pediatr. 2016 Apr;170(4):381-90. doi: 10.1001/jamapediatrics.2015.4430. JAMA Pediatr. 2016. PMID: 26882542 Free PMC article.
-
Dietary acrylamide intake during pregnancy and postnatal growth and obesity: Results from the Norwegian Mother and Child Cohort Study (MoBa).Environ Int. 2018 Apr;113:325-334. doi: 10.1016/j.envint.2018.01.004. Epub 2018 Feb 3. Environ Int. 2018. PMID: 29398013
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Impacts of Caffeine during Pregnancy.Trends Endocrinol Metab. 2020 Mar;31(3):218-227. doi: 10.1016/j.tem.2019.11.004. Epub 2019 Dec 6. Trends Endocrinol Metab. 2020. PMID: 31818639 Free PMC article. Review.
-
Maternal, but not paternal or grandparental, caffeine intake is associated with childhood obesity and adiposity: The Lifeways Cross-Generation Cohort Study.Am J Clin Nutr. 2019 Jun 1;109(6):1648-1655. doi: 10.1093/ajcn/nqz019. Am J Clin Nutr. 2019. PMID: 31136661 Free PMC article.
-
Association of maternal caffeine intake during pregnancy with low birth weight, childhood overweight, and obesity: a meta-analysis of cohort studies.Int J Obes (Lond). 2021 Feb;45(2):279-287. doi: 10.1038/s41366-020-0617-4. Epub 2020 Jun 9. Int J Obes (Lond). 2021. PMID: 32518355 Review.
-
Diet quality of Norwegian children at 3 and 7 years: changes, predictors and longitudinal association with weight.Int J Obes (Lond). 2022 Jan;46(1):10-20. doi: 10.1038/s41366-021-00951-x. Epub 2021 Aug 30. Int J Obes (Lond). 2022. PMID: 34462565
-
Association of Maternal Caffeine Consumption During Pregnancy With Child Growth.JAMA Netw Open. 2022 Oct 3;5(10):e2239609. doi: 10.1001/jamanetworkopen.2022.39609. JAMA Netw Open. 2022. PMID: 36315142 Free PMC article.
References
-
- EFSA EFSA-PoDP Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA Journal 2015;13:4102.
-
- VKM. Risk assessment of "other substances"– Caffeine. Opinion of the Panel on Food Additives, Flavourings, Processing Aids, Materials in Contact with Food and Cosmetics of the Norwegian Scientific Committee for Food Safety. Oslo, Norway: VKM, 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
