Cryo-electron microscopy structures and progress toward a dynamic understanding of KATP channels
- PMID: 29685928
- PMCID: PMC5940251
- DOI: 10.1085/jgp.201711978
Cryo-electron microscopy structures and progress toward a dynamic understanding of KATP channels
Abstract
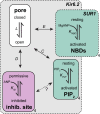
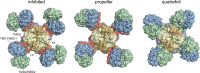
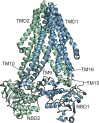
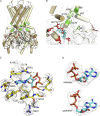
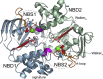
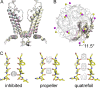
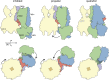
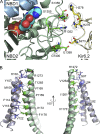
Adenosine triphosphate (ATP)-sensitive K+ (KATP) channels are molecular sensors of cell metabolism. These hetero-octameric channels, comprising four inward rectifier K+ channel subunits (Kir6.1 or Kir6.2) and four sulfonylurea receptor (SUR1 or SUR2A/B) subunits, detect metabolic changes via three classes of intracellular adenine nucleotide (ATP/ADP) binding site. One site, located on the Kir subunit, causes inhibition of the channel when ATP or ADP is bound. The other two sites, located on the SUR subunit, excite the channel when bound to Mg nucleotides. In pancreatic β cells, an increase in extracellular glucose causes a change in oxidative metabolism and thus turnover of adenine nucleotides in the cytoplasm. This leads to the closure of KATP channels, which depolarizes the plasma membrane and permits Ca2+ influx and insulin secretion. Many of the molecular details regarding the assembly of the KATP complex, and how changes in nucleotide concentrations affect gating, have recently been uncovered by several single-particle cryo-electron microscopy structures of the pancreatic KATP channel (Kir6.2/SUR1) at near-atomic resolution. Here, the author discusses the detailed picture of excitatory and inhibitory ligand binding to KATP that these structures present and suggests a possible mechanism by which channel activation may proceed from the ligand-binding domains of SUR to the channel pore.
© 2018 Puljung.
Figures





References
-
- Aguilar-Bryan L., Nichols C.G., Wechsler S.W., Clement J.P. IV, Boyd A.E. III, González G., Herrera-Sosa H., Nguy K., Bryan J., and Nelson D.A.. 1995. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 268:423–426. 10.1126/science.7716547 - DOI - PubMed
-
- Arkhammar P., Nilsson T., Rorsman P., and Berggren P.O.. 1987. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J. Biol. Chem. 262:5448–5454. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

