Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort
- PMID: 29685952
- PMCID: PMC5916007
- DOI: 10.1182/bloodadvances.2018016121
Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort
Abstract
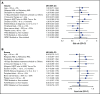
Although hypomethylating agents (HMAs) are frequently used in the frontline treatment of older acute myeloid leukemia (AML) patients, little is known about their effectiveness in relapsed or primary treatment-refractory (RR)-AML. Using an international multicenter retrospective database, we studied the effectiveness of HMAs in RR-AML and evaluated for predictors of response and overall survival (OS). A total of 655 patients from 12 centers received azacitidine (57%) or decitabine (43%), including 290 refractory (44%) and 365 relapsed (56%) patients. Median age at diagnosis was 65 years. Best response to HMAs was complete remission (CR; 11%) or CR with incomplete count recovery (CRi; 5.3%). Additionally, 8.5% experienced hematologic improvement. Median OS was 6.7 months (95% confidence interval, 6.1-7.3). As expected, OS differed significantly by best response, with patients achieving CR and CRi having a median OS of 25.3 and 14.6 months, respectively. In multivariate analysis, the presence of ≤5% circulating blasts and a 10-day schedule of decitabine were associated with improved response rates, whereas the presence of >5% circulating blasts and >20% bone marrow blasts were associated with decreased OS. A significant subset of RR-AML patients (16%) achieved CR/CRi with HMAs and experienced a median OS of 21 months. Outside of a clinical trial, HMAs represent a reasonable therapeutic option for some patients with RR-AML.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.M. received honoraria and research funding from Celgene. R.I. received research funding from Janssen Pharmaceuticals and Novartis. E.K.R. is a consultant for Novartis, Incyte, Celgene, and Pfizer; a member of the speaker’s bureau for Incyte, Celgene, Novartis, and ARIAD; has received travel monies from Celgene and Novartis; has an advisory role for Incyte, Pfizer, and Celgene; and received institutional research funding from Pfizer, Astellas Pharma, Bristol-Myers Squibb, and NS Pharma. M.A.S. is on the Board of Directors or advisory committees for Celgene, Takeda, and Opsona. N.A.P. is a consultant for CTI BioPharma, Alexion Pharmaceuticals, ARIAD, and Incyte and received institutional research funding from Boehringer Ingelheim, Astellas Pharma, Daiichi Sankyo, Sunesis Pharmaceuticals, Celator, Pfizer, and Astex Pharmaceuticals. A.M.B. received institutional research funding from Celgene and Takeda. R.S.K. has stock or other ownership in AbbVie; has received travel monies from Celgene, Alexion Pharmaceuticals, and Incyte; has received honoraria from or is a consultant for Celgene and Novartis; is a member of the speaker’s bureau for Novartis and Alexion Pharmaceuticals; and has received institutional research funding from Celgene, GlaxoSmithKline, Eleos, Boehringer Ingelheim, and Incyte. A.A.-K. received institutional research funding from Novartis, Onconova Therapeutics, Celgene, Bristol-Myers Squibb, Astex Pharmaceuticals, and Ambit Biosciences. V.S. is a consultant for Janssen Pharmaceuticals, AbbVie, and Otsuka; has received honoraria from Celgene, Janssen Pharmaceuticals, and Novartis; has received research funding from Celgene; and is on the Board of Directors or advisory committees for AbbVie and Amgen. A.T.F. is a consultant for Seattle Genetics, Celgene, Agios, MedImmune, and Amgen; has received honoraria from Seattle Genetics, Celgene, Agios, and Pfizer; is on the Board of Directors or advisory committees for Seattle Genetics, Juno, Celgene, Agios, MedImmune, and Amgen; and has received research funding from Seattle Genetics, Takeda, and Celgene. G.J.R. is a consultant for AbbVie, Agios, Amgen, Amphivena, Array Biopharma, Astex Pharmaceuticals, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova Therapeutics, Pfizer, Roche, Boehringer Ingelheim, GlaxoSmithKline, Shire, Astex Pharmaceuticals, Cellectis, and Sunesis Pharmaceuticals; received research funding from Cellectis; received travel monies from AstraZeneca, Shire, Astellas Pharma, Celator, Incyte, Roche, Amphivena, MEI Pharma, Astex Pharmaceuticals, Janssen Pharmaceuticals, and Juno Therapeutics; and received institutional research funding from AbbVie, Agios, Astex Pharmaceuticals, Celgene, CTI BioPharma, Karyopharm Therapeutics, MedImmune, MEI Pharma, Moffitt, Novartis, Onconova Therapeutics, Pfizer, Sunesis Pharmaceuticals, Tensha Therapeutics, and Cellectis. P.F. received honoraria and research funding from Amgen, Astex, Celgene, and Janssen Pharmaceuticals. M.R.L. has received research funding from Amgen, Novartis, Astellas Pharma, and Actinium Pharmaceuticals; has received honoraria and travel monies from Amgen; and is a consultant for Amgen. N.V. received honoraria from and is a consultant for Amgen, Celgene, Novartis, Roche, and Servier. U.G. received honoraria and research funding from Celgene and Novartis and received honoraria from Janssen Pharmaceuticals. A.M.Z. received honoraria from and is a consultant for AbbVie, Ostuka, Pfizer, Gilead, Celgene, Ariad, Incyte, Agios, Novartis, Takeda, Daiichi Sankyo, and Boehringer Ingelheim; received honoraria from and is a member of the speaker’s bureau for Takeda; and received institutional research funding from Celgene, Pfizer, Incyte, ADC Therapeutics, Medimmune, Takeda, AbbVie, and Boehringer Ingelheim. The remaining authors declare no competing financial interests.
Figures



References
-
- Podoltsev NA, Stahl M, Zeidan AM, Gore SD. Selecting initial treatment of acute myeloid leukaemia in older adults. Blood Rev. 2017;31(2):43-62. - PubMed
-
- Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. - PubMed
-
- Büchner T, Berdel WE, Haferlach C, et al. . Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27(1):61-69. - PubMed
-
- Burnett AK, Milligan D, Prentice AG, et al. . A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-1124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

