Influence of Gestational Age at Initiation of Antihypertensive Therapy: Secondary Analysis of CHIPS Trial Data (Control of Hypertension in Pregnancy Study)
- PMID: 29686009
- PMCID: PMC5959211
- DOI: 10.1161/HYPERTENSIONAHA.117.10689
Influence of Gestational Age at Initiation of Antihypertensive Therapy: Secondary Analysis of CHIPS Trial Data (Control of Hypertension in Pregnancy Study)
Abstract
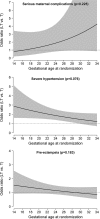
For hypertensive women in CHIPS (Control of Hypertension in Pregnancy Study), we assessed whether the maternal benefits of tight control could be achieved, while minimizing any potentially negative effect on fetal growth, by delaying initiation of antihypertensive therapy until later in pregnancy. For the 981 women with nonsevere, chronic or gestational hypertension randomized to less-tight (target diastolic blood pressure, 100 mm Hg), or tight (target, 85 mm Hg) control, we used mixed-effects logistic regression to examine whether the effect of less-tight (versus tight) control on major outcomes was dependent on gestational age at randomization, adjusting for baseline factors as in the primary analysis and including an interaction term between gestational age at randomization and treatment allocation. Gestational age was considered categorically (quartiles) and continuously (linear or quadratic form), and the optimal functional form selected to provide the best fit to the data based on the Akaike information criterion. Randomization before (but not after) 24 weeks to less-tight (versus tight) control was associated with fewer babies with birth weight <10th centile (Pinteraction=0.005), but more preterm birth (Pinteraction=0.043), and no effect on perinatal death or high-level neonatal care >48 hours (Pinteraction=0.354). For the mother, less-tight (versus tight) control was associated with more severe hypertension at all gestational ages but particularly so before 28 weeks (Pinteraction=0.076). In women with nonsevere, chronic, or gestational hypertension, there seems to be no gestational age at which less-tight (versus tight) control is the preferred management strategy to optimize maternal or perinatal outcomes.
Clinical trial registration: URL: https://www.isrctn.com. Unique identifier: ISRCTN71416914.
Keywords: blood pressure; fetal growth restriction; humans; hypertension, pregnancy-induced; preeclampsia; pregnancy outcome.
© 2018 The Authors.
Figures

References
-
- Magee LA, von Dadelszen P, Stones W. The FIGO Textbook of Pregnancy Hypertension–An Evidence-Based Guide to Monitoring, Prevention and Management. London, UK: The Global Library of Women’s Medicine 2016; http://www.glowm.com.
-
- Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014:CD002252. - PubMed
-
- von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet. 2000;355:87–92. - PubMed
-
- Nakhai-Pour HR, Rey E, Bérard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Res B Dev Reprod Toxicol. 2010;89:147–154. doi: 10.1002/bdrb.20238. - PubMed
-
- Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

