Homologous Recombination and Replication Fork Protection: BRCA2 and More!
- PMID: 29686033
- PMCID: PMC6333483
- DOI: 10.1101/sqb.2017.82.035006
Homologous Recombination and Replication Fork Protection: BRCA2 and More!
Abstract
BRCA2 is a breast and ovarian tumor suppressor that guards against genome instability, a hallmark of cancer. Significant progress has been made in improving our understanding of BRCA2 function from biochemical, cellular, and mouse studies. The knowledge gained has been actively exploited to develop therapeutic strategies, including PARP inhibition, which has shown promising clinical outcomes. Recently, tremendous excitement has been generated by the findings of the roles of BRCA2 and other proteins in suppressing replication stress through homologous recombination and in the protection of stalled replication forks. Processes such as mitotic DNA synthesis and fork reversal have taken center stage in these studies. Here, we discuss our recent findings in the context of these advances.
© 2017 Feng and Jasin; Published by Cold Spring Harbor Laboratory Press.
Figures
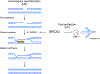



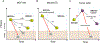
References
-
- Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R, Wahl GM. 1998. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol 8: 145–155. - PubMed
-
- Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg  et al. 2003. Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. The American Journal of Human Genetics 72: 1117–1130. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
