Innate Immunity to Enteric Hepatitis Viruses
- PMID: 29686040
- PMCID: PMC6396338
- DOI: 10.1101/cshperspect.a033464
Innate Immunity to Enteric Hepatitis Viruses
Abstract
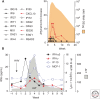
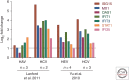
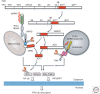
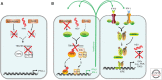
Although hepatitis A virus (HAV) and hepatitis E virus (HEV) are both positive-strand RNA viruses that replicate in the cytoplasm of hepatocytes, there are important differences in the ways they induce and counteract host innate immune responses. HAV is remarkably stealthy because of its ability to evade and disrupt innate signaling pathways that lead to interferon production. In contrast, HEV does not block interferon production. Instead, it persists in the presence of an interferon response. These differences may provide insight into HEV persistence in immunocompromised patients, an emerging health problem in developed countries.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Allweiss L, Gass S, Giersch K, Groth A, Kah J, Volz T, Rapp G, Schobel A, Lohse AW, Polywka S, et al. 2016. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J Hepatol 64: 1033–1040. - PubMed
-
- Asher LVS, Binn LN, Mensing TL, Marchwicki RH, Vassell RA, Young GD. 1995. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivergatus). J Med Virol 47: 260–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
