RAPTOR Controls Developmental Growth Transitions by Altering the Hormonal and Metabolic Balance
- PMID: 29686055
- PMCID: PMC6001337
- DOI: 10.1104/pp.17.01711
RAPTOR Controls Developmental Growth Transitions by Altering the Hormonal and Metabolic Balance
Abstract
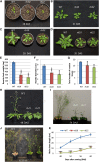
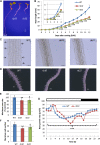
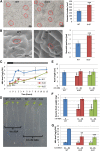
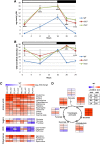
Vegetative growth requires the systemic coordination of numerous cellular processes, which are controlled by regulatory proteins that monitor extracellular and intracellular cues and translate them into growth decisions. In eukaryotes, one of the central factors regulating growth is the serine/threonine protein kinase Target of Rapamycin (TOR), which forms complexes with regulatory proteins. To understand the function of one such regulatory protein, Regulatory-Associated Protein of TOR 1B (RAPTOR1B), in plants, we analyzed the effect of raptor1b mutations on growth and physiology in Arabidopsis (Arabidopsis thaliana) by detailed phenotyping, metabolomic, lipidomic, and proteomic analyses. Mutation of RAPTOR1B resulted in a strong reduction of TOR kinase activity, leading to massive changes in central carbon and nitrogen metabolism, accumulation of excess starch, and induction of autophagy. These shifts led to a significant reduction of plant growth that occurred nonlinearly during developmental stage transitions. This phenotype was accompanied by changes in cell morphology and tissue anatomy. In contrast to previous studies in rice (Oryza sativa), we found that the Arabidopsis raptor1b mutation did not affect chloroplast development or photosynthetic electron transport efficiency; however, it resulted in decreased CO2 assimilation rate and increased stomatal conductance. The raptor1b mutants also had reduced abscisic acid levels. Surprisingly, abscisic acid feeding experiments resulted in partial complementation of the growth phenotypes, indicating the tight interaction between TOR function and hormone synthesis and signaling in plants.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures







Similar articles
-
Brassinosteroids modulate autophagy through phosphorylation of RAPTOR1B by the GSK3-like kinase BIN2 in Arabidopsis.Autophagy. 2023 Apr;19(4):1293-1310. doi: 10.1080/15548627.2022.2124501. Epub 2022 Sep 24. Autophagy. 2023. PMID: 36151786 Free PMC article.
-
Regulatory-associated protein of TOR (RAPTOR) alters the hormonal and metabolic composition of Arabidopsis seeds, controlling seed morphology, viability and germination potential.Plant J. 2017 Nov;92(4):525-545. doi: 10.1111/tpj.13667. Epub 2017 Sep 30. Plant J. 2017. PMID: 28845535
-
Regulatory-Associated Protein of TOR 1B (RAPTOR1B) regulates hormonal switches during seed germination in Arabidopsis thaliana.Plant Signal Behav. 2019;14(7):1613130. doi: 10.1080/15592324.2019.1613130. Epub 2019 May 6. Plant Signal Behav. 2019. PMID: 31058576 Free PMC article.
-
Mutation in the Arabidopsis regulatory-associated protein TOR 1B (RAPTOR1B) leads to decreased jasmonates levels in leaf tissue.Plant Signal Behav. 2019;14(10):e1649567. doi: 10.1080/15592324.2019.1649567. Epub 2019 Aug 5. Plant Signal Behav. 2019. PMID: 31382813 Free PMC article.
-
Photosynthesis of root chloroplasts developed in Arabidopsis lines overexpressing GOLDEN2-LIKE transcription factors.Plant Cell Physiol. 2013 Aug;54(8):1365-77. doi: 10.1093/pcp/pct086. Epub 2013 Jun 7. Plant Cell Physiol. 2013. PMID: 23749810 Free PMC article.
Cited by
-
TORC pathway intersects with a calcium sensor kinase network to regulate potassium sensing in Arabidopsis.Proc Natl Acad Sci U S A. 2023 Nov 21;120(47):e2316011120. doi: 10.1073/pnas.2316011120. Epub 2023 Nov 15. Proc Natl Acad Sci U S A. 2023. PMID: 37967217 Free PMC article.
-
Complex Changes in Membrane Lipids Associated with the Modification of Autophagy in Arabidopsis.Metabolites. 2022 Feb 18;12(2):190. doi: 10.3390/metabo12020190. Metabolites. 2022. PMID: 35208263 Free PMC article.
-
Modulation of cell differentiation and growth underlies the shift from bud protection to light capture in cauline leaves.Plant Physiol. 2024 Oct 1;196(2):1214-1230. doi: 10.1093/plphys/kiae408. Plant Physiol. 2024. PMID: 39106417 Free PMC article.
-
Brassinosteroids modulate autophagy through phosphorylation of RAPTOR1B by the GSK3-like kinase BIN2 in Arabidopsis.Autophagy. 2023 Apr;19(4):1293-1310. doi: 10.1080/15548627.2022.2124501. Epub 2022 Sep 24. Autophagy. 2023. PMID: 36151786 Free PMC article.
-
Nitric oxide-an antidote to seed aging modifies meta-tyrosine content and expression of aging-linked genes in apple embryos.Front Plant Sci. 2022 Aug 30;13:929245. doi: 10.3389/fpls.2022.929245. eCollection 2022. Front Plant Sci. 2022. PMID: 36110361 Free PMC article.
References
-
- Ache P, Bauer H, Kollist H, Al-Rasheid KA, Lautner S, Hartung W, Hedrich R (2010) Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J 62: 1072–1082 - PubMed
-
- Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2: 875–877 - PubMed
-
- Albus CA, Salinas A, Czarnecki O, Kahlau S, Rothbart M, Thiele W, Lein W, Bock R, Grimm B, Schöttler MA (2012) LCAA, a novel factor required for magnesium protoporphyrin monomethylester cyclase accumulation and feedback control of aminolevulinic acid biosynthesis in tobacco. Plant Physiol 160: 1923–1939 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

