Neuronal activity regulates neurotransmitter switching in the adult brain following light-induced stress
- PMID: 29686073
- PMCID: PMC5960321
- DOI: 10.1073/pnas.1801598115
Neuronal activity regulates neurotransmitter switching in the adult brain following light-induced stress
Abstract
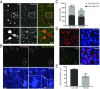
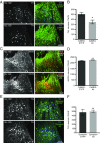
Neurotransmitter switching in the adult mammalian brain occurs following photoperiod-induced stress, but the mechanism of regulation is unknown. Here, we demonstrate that elevated activity of dopaminergic neurons in the paraventricular nucleus of the hypothalamus (PaVN) in the adult rat is required for the loss of dopamine expression after long-day photoperiod exposure. The transmitter switch occurs exclusively in PaVN dopaminergic neurons that coexpress vesicular glutamate transporter 2 (VGLUT2), is accompanied by a loss of dopamine type 2 receptors (D2Rs) on corticotrophin-releasing factor (CRF) neurons, and can lead to increased release of CRF. Suppressing activity of all PaVN glutamatergic neurons decreases the number of inhibitory PaVN dopaminergic neurons, indicating homeostatic regulation of transmitter expression in the PaVN.
Keywords: dopaminergic neurons; paraventricular nucleus of the hypothalamus; stress response; transmitter coexpression; transmitter switching.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Neurotransmitter switching in the adult brain regulates behavior.Science. 2013 Apr 26;340(6131):449-53. doi: 10.1126/science.1234152. Science. 2013. PMID: 23620046
-
Photoperiod-induced neurotransmitter plasticity declines with aging: An epigenetic regulation?J Comp Neurol. 2020 Feb 1;528(2):199-210. doi: 10.1002/cne.24747. Epub 2019 Aug 1. J Comp Neurol. 2020. PMID: 31343079
-
Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain.J Comp Neurol. 2006 Oct 10;498(5):581-92. doi: 10.1002/cne.21054. J Comp Neurol. 2006. PMID: 16917821
-
Molecular diversity of corticotropin-releasing hormone mRNA-containing neurons in the hypothalamus.J Endocrinol. 2017 Mar;232(3):R161-R172. doi: 10.1530/JOE-16-0256. Epub 2017 Jan 5. J Endocrinol. 2017. PMID: 28057867 Review.
-
Dopaminergic system and neurons: Role in multiple neurological diseases.Neuropharmacology. 2024 Dec 1;260:110133. doi: 10.1016/j.neuropharm.2024.110133. Epub 2024 Aug 27. Neuropharmacology. 2024. PMID: 39197818 Review.
Cited by
-
A dynamic role for dopamine receptors in the control of mammalian spinal networks.Sci Rep. 2020 Oct 2;10(1):16429. doi: 10.1038/s41598-020-73230-w. Sci Rep. 2020. PMID: 33009442 Free PMC article.
-
A neural circuit perspective on brain aromatase.Front Neuroendocrinol. 2022 Apr;65:100973. doi: 10.1016/j.yfrne.2021.100973. Epub 2021 Dec 21. Front Neuroendocrinol. 2022. PMID: 34942232 Free PMC article. Review.
-
Drug-induced change in transmitter identity is a shared mechanism generating cognitive deficits.Res Sq [Preprint]. 2023 Dec 13:rs.3.rs-3689243. doi: 10.21203/rs.3.rs-3689243/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Sep 26;15(1):8260. doi: 10.1038/s41467-024-52451-x. PMID: 38168375 Free PMC article. Updated. Preprint.
-
QnAs with Nicholas C. Spitzer.Proc Natl Acad Sci U S A. 2018 May 15;115(20):5047-5048. doi: 10.1073/pnas.1805781115. Epub 2018 Apr 23. Proc Natl Acad Sci U S A. 2018. PMID: 29686066 Free PMC article. No abstract available.
-
Bioelectronic Medicine: a multidisciplinary roadmap from biophysics to precision therapies.Front Integr Neurosci. 2024 Feb 19;18:1321872. doi: 10.3389/fnint.2024.1321872. eCollection 2024. Front Integr Neurosci. 2024. PMID: 38440417 Free PMC article. Review.
References
-
- Bains JS, Wamsteeker Cusulin JI, Inoue W. Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci. 2015;16:377–388. - PubMed
-
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–453. - PubMed
-
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

