Elucidation of the trigonelline degradation pathway reveals previously undescribed enzymes and metabolites
- PMID: 29686076
- PMCID: PMC5948990
- DOI: 10.1073/pnas.1722368115
Elucidation of the trigonelline degradation pathway reveals previously undescribed enzymes and metabolites
Abstract
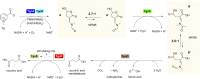
Trigonelline (TG; N-methylnicotinate) is a ubiquitous osmolyte. Although it is known that it can be degraded, the enzymes and metabolites have not been described so far. In this work, we challenged the laboratory model soil-borne, gram-negative bacterium Acinetobacter baylyi ADP1 (ADP1) for its ability to grow on TG and we identified a cluster of catabolic, transporter, and regulatory genes. We dissected the pathway to the level of enzymes and metabolites, and proceeded to in vitro reconstruction of the complete pathway by six purified proteins. The four enzymatic steps that lead from TG to methylamine and succinate are described, and the structures of previously undescribed metabolites are provided. Unlike many aromatic compounds that undergo hydroxylation prior to ring cleavage, the first step of TG catabolism proceeds through direct cleavage of the C5-C6 bound, catalyzed by a flavin-dependent, two-component oxygenase, which yields (Z)-2-((N-methylformamido)methylene)-5-hydroxy-butyrolactone (MFMB). MFMB is then oxidized into (E)-2-((N-methylformamido) methylene) succinate (MFMS), which is split up by a hydrolase into carbon dioxide, methylamine, formic acid, and succinate semialdehyde (SSA). SSA eventually fuels up the TCA by means of an SSA dehydrogenase, assisted by a Conserved Hypothetical Protein. The cluster is conserved across marine, soil, and plant-associated bacteria. This emphasizes the role of TG as a ubiquitous nutrient for which an efficient microbial catabolic toolbox is available.
Keywords: LC/MS-Orbitrap; N-heterocycle degradation; bacterial metabolism; functional genomics; trigonelline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bastard K, et al. Parallel evolution of non-homologous isofunctional enzymes in methionine biosynthesis. Nat Chem Biol. 2017;13:858–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

