Mitochondrial DNA induces Foley catheter related bladder inflammation via Toll-like receptor 9 activation
- PMID: 29686303
- PMCID: PMC5913242
- DOI: 10.1038/s41598-018-24818-w
Mitochondrial DNA induces Foley catheter related bladder inflammation via Toll-like receptor 9 activation
Abstract
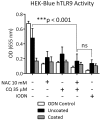
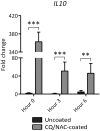
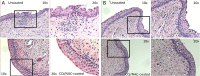
Bladder instrumentation engages the innate immune system via neutrophil activation, promoting inflammation and pain. Elevated levels of mitochondrial DNA (mtDNA) have been associated with tissue damage and organ dysfunction. We hypothesized that local bladder trauma induced by a Foley catheter (FC) will result in mtDNA release, migration of neutrophils into the bladder lumen, and activation of the Toll-like receptor 9 (TLR9) and nuclear factor kappa B (NF-κB) pathway leading to bladder tissue damage. We randomized 10 swine into two groups receiving uncoated, or chloroquine/N-Acetylcysteine (CQ/NAC)-coated FCs. Urine samples were analyzed for mtDNA activation of TLR9/NF-κB as demonstrated by indicators of neutrophil adhesion, migration, and activation. We found that uncoated FCs resulted in a unique active neutrophil phenotype that correlated with bladder epithelial injury, neutrophilia, necrosis, mtDNA release, TLR9/NF-κB activation, transcription and secretion of pro-inflammatory cytokines, and enhanced respiratory burst. In our study we observed that the high levels of mtDNA and elevated TLR9/NF-κB activity were ameliorated in the CQ/NAC-coated FC group. These findings suggest that post-migrated bladder luminal neutrophils are involved in local tissue damage and amelioration of the mtDNA/TLR9/NF-κB inflammatory axis may represent a therapeutic target to prevent inflammation, and bladder tissue injury.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Peychl L, Zalud R. Changes in the urinary bladder caused by short-term permanent catheter insertion. Cas. Lek. Cesk. 2008;147:325–339. - PubMed
-
- Shahin RD, et al. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J. Immunol. 1987;138:3475–3480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

