IGF-Binding Proteins: Why Do They Exist and Why Are There So Many?
- PMID: 29686648
- PMCID: PMC5900387
- DOI: 10.3389/fendo.2018.00117
IGF-Binding Proteins: Why Do They Exist and Why Are There So Many?
Abstract
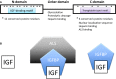
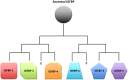
Insulin-like growth factors (IGFs) are key growth-promoting peptides that act as both endocrine hormones and autocrine/paracrine growth factors. In the bloodstream and in local tissues, most IGF molecules are bound by one of the members of the IGF-binding protein (IGFBP) family, of which six distinct types exist. These proteins bind to IGF with an equal or greater affinity than the IGF1 receptor and are thus in a key position to regulate IGF signaling globally and locally. Binding to an IGFBP increases the half-life of IGF in the circulation and blocks its potential binding to the insulin receptor. In addition to these classical roles, IGFBPs have been shown to modulate IGF signaling locally under various conditions. Although members of the IGFBP family share significant sequence homology, they each have unique structural features and play distinct roles. These IGFBP genes also have different modes of regulation and distinct expression patterns. Some IGFBPs have been found to bind to their own receptors or to translocate into the interior compartments of cells where they may execute IGF-independent actions. In spite of this functional and regulatory diversity, it has been puzzling that loss-of-function studies have yielded relatively little information about the physiological functions of IGFBPs. In this review, we suggest that evolution has tended to retain an array of IGFBPs in order to facilitate fine-tuning of IGF signaling. We explore the emerging explanation that many IGFBP functions have evolved to allow the targeted adjustment of IGF signaling under stressful or irregular conditions, which would likely not be revealed in a standard laboratory setting.
Keywords: evolution; insulin-like growth factor; insulin-like growth factor 1 receptor; insulin-like growth factor signaling; insulin-like growth factor-binding protein.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

