Antinociceptive Activity of Methanolic Extract of Clinacanthus nutans Leaves: Possible Mechanisms of Action Involved
- PMID: 29686743
- PMCID: PMC5857305
- DOI: 10.1155/2018/9536406
Antinociceptive Activity of Methanolic Extract of Clinacanthus nutans Leaves: Possible Mechanisms of Action Involved
Abstract
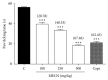
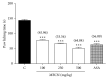
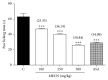
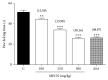
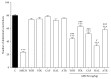
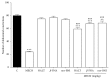
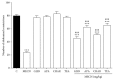
Methanolic extract of Clinacanthus nutans Lindau leaves (MECN) has been proven to possess antinociceptive activity that works via the opioid and NO-dependent/cGMP-independent pathways. In the present study, we aimed to further determine the possible mechanisms of antinociception of MECN using various nociceptive assays. The antinociceptive activity of MECN was (i) tested against capsaicin-, glutamate-, phorbol 12-myristate 13-acetate-, bradykinin-induced nociception model; (ii) prechallenged against selective antagonist of opioid receptor subtypes (β-funaltrexamine, naltrindole, and nor-binaltorphimine); (iii) prechallenged against antagonist of nonopioid systems, namely, α2-noradrenergic (yohimbine), β-adrenergic (pindolol), adenosinergic (caffeine), dopaminergic (haloperidol), and cholinergic (atropine) receptors; (iv) prechallenged with inhibitors of various potassium channels (glibenclamide, apamin, charybdotoxin, and tetraethylammonium chloride). The results demonstrated that the orally administered MECN (100, 250, and 500 mg/kg) significantly (p < 0.05) reversed the nociceptive effect of all models in a dose-dependent manner. Moreover, the antinociceptive activity of 500 mg/kg MECN was significantly (p < 0.05) inhibited by (i) antagonists of μ-, δ-, and κ-opioid receptors; (ii) antagonists of α2-noradrenergic, β-adrenergic, adenosinergic, dopaminergic, and cholinergic receptors; and (iii) blockers of different K+ channels (voltage-activated-, Ca2+-activated, and ATP-sensitive-K+ channels, resp.). In conclusion, MECN-induced antinociception involves modulation of protein kinase C-, bradykinin-, TRVP1 receptors-, and glutamatergic-signaling pathways; opioidergic, α2-noradrenergic, β-adrenergic, adenosinergic, dopaminergic, and cholinergic receptors; and nonopioidergic receptors as well as the opening of various K+ channels. The antinociceptive activity could be associated with the presence of several flavonoid-based bioactive compounds and their synergistic action with nonvolatile bioactive compounds.
Figures
References
-
- Bektas N., Nemutlu D., Ulugbay G., Arslan R. The role of muscarinic receptors in pain modulation. World Journal of Pharmaceutical and Medical Research. 2015;1(1):40–49.
-
- Zendehdel M., Taati M., Jadidoleslami M., Bashiri A. Evaluation of pharmacological mechanisms of antinociceptive effect of Teucrium polium on visceral pain in mice. Iranian Journal of Veterinary Research. 2011;12(4):292–297.
-
- Helms J. E., Barone C. P. Physiology and treatment of pain. Critical Care Nurse. 2008;28(6):38–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

