Secondary Degeneration of Auditory Neurons after Topical Aminoglycoside Administration in a Gerbil Model
- PMID: 29687008
- PMCID: PMC5852872
- DOI: 10.1155/2018/9158187
Secondary Degeneration of Auditory Neurons after Topical Aminoglycoside Administration in a Gerbil Model
Abstract
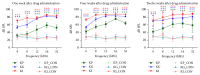
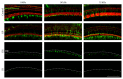
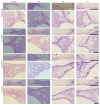
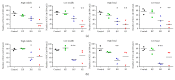
Hair cells in the cochlea can be damaged by various causes. Damaged hair cells can lead to additional destruction of parts of the auditory afferent pathway sequentially, which is called secondary degeneration. Recently, researches regarding cochlear implants have been actively carried out for clinical purposes; secondary degeneration in animals is a much more practical model for identifying the prognosis of cochlear implants. However, an appropriate model for this research is not established yet. Thus, we developed a secondary degeneration model using an ototoxic drug. 35 gerbils were separated into four different groups and kanamycin was applied via various approaches. ABR was measured several times after drug administration. SGCs were also counted to identify any secondary degeneration. The results showed that outer and inner HCs were damaged in all kanamycin-treated groups. Twelve weeks after kanamycin treatment, the round window membrane injection group showed severe subject differences in hair cells and SGC damage, whereas the gelfoam group showed consistent and severe damage in hair cells and SGCs. In this study, we successfully induced secondary degeneration in hair cells in a gerbil model. This model can be used for various purposes in the hearing research area either for treatment or for preservation.
Figures


Similar articles
-
Aminoglycoside ototoxicity and hair cell ablation in the adult gerbil: A simple model to study hair cell loss and regeneration.Hear Res. 2015 Jul;325:12-26. doi: 10.1016/j.heares.2015.03.002. Epub 2015 Mar 14. Hear Res. 2015. PMID: 25783988 Free PMC article.
-
Effects of aminoglycoside administration on cochlear elements in human temporal bones.Auris Nasus Larynx. 2004 Dec;31(4):383-8. doi: 10.1016/j.anl.2004.09.011. Auris Nasus Larynx. 2004. PMID: 15571911
-
Kanamycin Damages Early Postnatal, but Not Adult Spiral Ganglion Neurons.Neurotox Res. 2017 Nov;32(4):603-613. doi: 10.1007/s12640-017-9773-2. Epub 2017 Jun 27. Neurotox Res. 2017. PMID: 28656549 Free PMC article.
-
Relations between cochlear histopathology and hearing loss in experimental cochlear implantation.Hear Res. 2013 Apr;298:27-35. doi: 10.1016/j.heares.2013.01.012. Epub 2013 Feb 5. Hear Res. 2013. PMID: 23396095
-
Stem cell-based approaches: Possible route to hearing restoration?World J Stem Cells. 2020 Jun 26;12(6):422-437. doi: 10.4252/wjsc.v12.i6.422. World J Stem Cells. 2020. PMID: 32742560 Free PMC article. Review.
Cited by
-
Distribution and Afferent Effects of Transplanted mESCs on Cochlea in Acute and Chronic Neural Hearing Loss Models.Biomed Res Int. 2021 Jun 21;2021:4956404. doi: 10.1155/2021/4956404. eCollection 2021. Biomed Res Int. 2021. PMID: 34250085 Free PMC article.
-
Applications of photobiomodulation in hearing research: from bench to clinic.Biomed Eng Lett. 2019 Jun 18;9(3):351-358. doi: 10.1007/s13534-019-00114-y. eCollection 2019 Aug. Biomed Eng Lett. 2019. PMID: 31456894 Free PMC article. Review.
References
-
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Annals of Otology, Rhinology & Laryngology. 1984;93(4):76–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

