Axonal abnormalities in vanishing white matter
- PMID: 29687020
- PMCID: PMC5899913
- DOI: 10.1002/acn3.540
Axonal abnormalities in vanishing white matter
Abstract
Objective: We aimed to study the occurrence and development of axonal pathology and the influence of astrocytes in vanishing white matter.
Methods: Axons and myelin were analyzed using electron microscopy and immunohistochemistry on Eif2b4 and Eif2b5 single- and double-mutant mice and patient brain tissue. In addition, astrocyte-forebrain co-culture studies were performed.
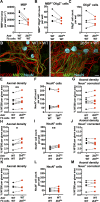
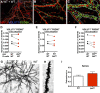
Results: In the corpus callosum of Eif2b5-mutant mice, myelin sheath thickness, axonal diameter, and G-ratio developed normally up to 4 months. At 7 months, however, axons had become thinner, while in control mice axonal diameters had increased further. Myelin sheath thickness remained close to normal, resulting in an abnormally low G-ratio in Eif2b5-mutant mice. In more severely affected Eif2b4-Eif2b5 double-mutants, similar abnormalities were already present at 4 months, while in milder affected Eif2b4 mutants, few abnormalities were observed at 7 months. Additionally, from 2 months onward an increased percentage of thin, unmyelinated axons and increased axonal density were present in Eif2b5-mutant mice. Co-cultures showed that Eif2b5 mutant astrocytes induced increased axonal density, also in control forebrain tissue, and that control astrocytes induced normal axonal density, also in mutant forebrain tissue. In vanishing white matter patient brains, axons and myelin sheaths were thinner than normal in moderately and severely affected white matter. In mutant mice and patients, signs of axonal transport defects and cytoskeletal abnormalities were minimal.
Interpretation: In vanishing white matter, axons are initially normal and atrophy later. Astrocytes are central in this process. If therapy becomes available, axonal pathology may be prevented with early intervention.
Figures






References
-
- van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol 2006;5:413–423. - PubMed
-
- Fogli A, Schiffmann R, Bertini E, et al. The effect of genotype on the natural history of eIF2B‐related leukodystrophies. Neurology 2004;62:1509–1517. - PubMed
-
- Hanefeld F, Holzbach U, Kruse B, et al. Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics 1993;24:244–248. - PubMed
-
- Schiffmann R, Moller JR, Trapp BD, et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol 1994;35:331–340. - PubMed
-
- van der Knaap MS, Barth PG, Gabreëls FJ, et al. A new leukoencephalopathy with vanishing white matter. Neurology 1997;48:845–855. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

