Meta-inflammation and cardiometabolic disease in obesity: Can heat therapy help?
- PMID: 29687041
- PMCID: PMC5902218
- DOI: 10.1080/23328940.2017.1384089
Meta-inflammation and cardiometabolic disease in obesity: Can heat therapy help?
Abstract
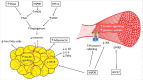
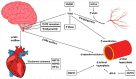
Obesity and associated metabolic dysfunction have reached epidemic proportions worldwide. The current theory linking metabolic disease and obesity involves ischemic adipose tissue initiating an inflammatory cascade that results in systemic insulin resistance and may eventually lead to type II diabetes mellitus. Diabetes and associated metabolic dysfunction increase the risk of developing cardiovascular disease and fatal cardiovascular events. By targeting key steps in this process, ischemia and inflammation, this cascade may be prevented or reversed and thus metabolic and cardiovascular health may be preserved in obesity. Regular heat exposure (termed 'heat therapy') offers potential to improve cardiometabolic health in obese individuals through a variety of mechanisms that include but are not limited to heat shock proteins, hypoxia-inducible factor 1α, and hemodynamic effects. The purpose of this review is to highlight the cardiometabolic decline in obese individuals stemming from adipose tissue dysfunction, and examine the ways in which heat therapy and associated cellular and systemic adaptations can intersect with this decline in function to improve or restore cardiovascular and metabolic health.
Keywords: cardiovascular disease; diabetes mellitus; heat acclimation; heat shock protein; hyperthermic conditioning; insulin resistance; sympathetic activity; thermotherapy.
Figures

References
-
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013;131(131):1–8. - PubMed
-
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: A systematic review and meta-analysis. Surg. 2004;292(14):1724–37. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
