Psychosocial Stress Exposure Disrupts Mammary Gland Development
- PMID: 29687293
- PMCID: PMC6207373
- DOI: 10.1007/s10911-018-9392-4
Psychosocial Stress Exposure Disrupts Mammary Gland Development
Abstract
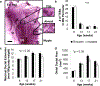
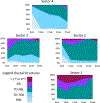
Exposure to psychosocial stressors and ensuing stress physiology have been associated with spontaneous invasive mammary tumors in the Sprague-Dawley rat model of human breast cancer. Mammary gland (MG) development is a time when physiologic and environmental exposures influence breast cancer risk. However, the effect of psychosocial stress exposure on MG development remains unknown. Here, in the first comprehensive longitudinal study of MG development in nulliparous female rats (from puberty through young adulthood; 8-25 wks of age), we quantify the spatial gradient of differentiation within the MG of socially stressed (isolated) and control (grouped) rats. We then demonstrate that social isolation increased stress reactivity to everyday stressors, resulting in downregulation of glucocorticoid receptor (GR) expression in the MG epithelium. Surprisingly, given that chemical carcinogens increase MG cancer risk by preventing normal terminal end bud (TEB) differentiation, chronic isolation stress did not alter TEBs. Instead, isolation blunted MG growth and alveolobular differentiation and reduced epithelial cell proliferation in these structures. Social isolation also enhanced corpora luteal progesterone at all ages but reduced estrogenization only in early adulthood, a pattern that precludes modulated ovarian function as a sufficient mechanism for the effects of isolation on MG development. This longitudinal study of natural variation provides an integrated view of MG development and the importance of increased GR activation in nulliparous ductal growth and alveolobular differentiation. Thus, social isolation and its physiological sequelae disrupt MG growth and differentiation and suggest a contribution of stress exposure during puberty and young adulthood to the previously observed increase in invasive MG cancer observed in chronically socially-isolated adult Sprague-Dawley rats.
Keywords: Alveolobular development; Mammary gland; Puberty; Stress; Terminal end buds; Young adulthood.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD et al. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci U S A 2009;106(52):22393–8. doi:10.1073/pnas.0910753106. - DOI - PMC - PubMed
-
- Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res 2005;11(2 Pt 2):931s-6s. - PubMed
-
- Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev 1995;4(5):567–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

