Semaglutide as a therapeutic option for elderly patients with type 2 diabetes: Pooled analysis of the SUSTAIN 1-5 trials
- PMID: 29687620
- PMCID: PMC6099273
- DOI: 10.1111/dom.13331
Semaglutide as a therapeutic option for elderly patients with type 2 diabetes: Pooled analysis of the SUSTAIN 1-5 trials
Abstract
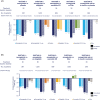
The efficacy and safety of semaglutide vs comparators in non-elderly (<65 years) and elderly (≥65 years) patients with type 2 diabetes (T2D) across the SUSTAIN 1-5 trials were evaluated. Patients were randomized to once-weekly subcutaneous semaglutide (0.5 or 1.0 mg) vs placebo, sitagliptin, exenatide or insulin. The primary objective was change in HbA1c and secondary objectives were changes in body weight and safety. Mean HbA1c decreased from baseline by 1.2%-1.5% and 1.5%-1.9% vs 0%-0.9% (non-elderly, n = 3045) and by 1.3%-1.5% and 1.2%-1.8% vs 0.2%-1.0% (elderly, n = 854) with semaglutide 0.5 and 1.0 mg vs comparators. Similar reductions from baseline in mean body weight with semaglutide occurred in both age groups. Similar proportions of patients experienced adverse events; premature treatment discontinuations were higher in elderly vs non-elderly patients. No increased risk of severe or blood glucose-confirmed hypoglycaemia was seen with semaglutide vs comparators between age groups. Semaglutide had a comparable efficacy and safety profile in non-elderly and elderly patients across the SUSTAIN 1-5 trials, making it an effective treatment option for elderly patients with T2D.
Keywords: GLP-1 analogue; antidiabetic drug; glycaemic control; incretin therapy; type 2 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
M. W. has received research support from Novo Nordisk, Eli Lilly, Janssen, Shire, Pfizer, Mylan and Sanofi; is on the advisory panel of Novo Nordisk, Sanofi and Eli Lilly; and is on the speaker's bureau of Novo Nordisk, Eli Lilly, Janssen, AstraZeneca, Sanofi, Merck, Shire and Mannkind. L. C. is a consultant for Novo Nordisk and is on the advisory board of Intarcia Therapeutics. D. T. is on the advisory panel of Novo Nordisk and has received research support from Novo Nordisk, Sanofi Aventis and Sanofi. G. N. and N. W. are full‐time employees of Novo Nordisk. B. C. is a consultant for Novo Nordisk; has received research support from Pfizer, Sanofi and Regeneron; and has received honoraria from Amgen, Pierre Fabre, Eli Lilly, MSD, Merck & Co, Novo Nordisk, Regeneron and Sanofi.
Figures
References
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. - PubMed
-
- Noale M, Veronese N, Cavallo Perin P, et al. Polypharmacy in elderly patients with type 2 diabetes receiving oral antidiabetic treatment. Acta Diabetol. 2016;53:323‐330. - PubMed
-
- Bailey CJ, Aschner P, Del Prato S, et al. Individualized glycaemic targets and pharmacotherapy in type 2 diabetes. Diab Vasc Dis Res. 2013;10:397‐409. - PubMed
-
- American Diabetes Association . Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1‐S135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

