Overexpression of Myosin Phosphatase Target Subunit 1 (MYPT1) Inhibits Tumor Progression and Metastasis of Gastric Cancer
- PMID: 29687789
- PMCID: PMC5937360
- DOI: 10.12659/msm.906852
Overexpression of Myosin Phosphatase Target Subunit 1 (MYPT1) Inhibits Tumor Progression and Metastasis of Gastric Cancer
Abstract
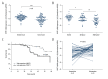
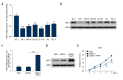
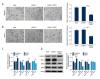
BACKGROUND Myosin phosphatase target subunit 1 (MYPT1) serves as a subgroup of myosin phosphatases, and is frequently low-expressed in human cancers. However, little is known about the effects of MYPT1 in gastric cancer (GC). MATERIAL AND METHODS In our study, MYPT1 expression was detected by quantitative real-time reverse transcription PCR (qRT-PCR) in GC tissues, different advanced pathological stages of GC tissues, and preoperative and postoperative patients. Kaplan-Meier analysis was used to measure the overall survival of GC patients. MYPT1 expression was analyzed by qRT-PCR and Western blot assays in GES-1 cells and GC cells. Cell proliferation, cycle, and migration and invasion abilities were detected by CCK-8, flow cytometry, and Transwell assays. E-cadherin, TIMP-2, MMP-2, MMP-9 RhoA, and p-RhoA expressions were assessed by qRT-PCR and Western blot assays in treated SNU-5 cells. RESULTS Our results indicated that MYPT1 was down-regulated in GC tissues and cells, and is related to clinical stages and overall survival of GC. Functional research demonstrated that overexpression of MYPT1 can inhibit cell proliferation, cell cycle progression, and migration and invasion of GC cells. Many studies on mechanisms reported that overexpression of MYPT1 dramatically improved the expression levels of cell cycle-related genes (Cyclin D1 and c-myc), significantly increased epithelial marker (E-cadherin) expression, and decreased invasion-associated genes (TIMP-2 and MMP-2) expressions in SNU-5 cells. In addition, we found that MYPT1 suppressed RhoA phosphorylation. CONCLUSIONS We verified that MYPT1 inhibits GC cell proliferation and metastasis by regulating RhoA phosphorylation.
Conflict of interest statement
None.
Figures


References
-
- Guo X, Guo L, Ji J, et al. miRNA-331-3p directly targets E2F1 and induces growth arrest in human gastric cancer. Biochem Biophys Res Commun. 2010;398(1):1–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

