Brain properties predict proximity to symptom onset in sporadic Alzheimer's disease
- PMID: 29688388
- PMCID: PMC5972641
- DOI: 10.1093/brain/awy093
Brain properties predict proximity to symptom onset in sporadic Alzheimer's disease
Abstract
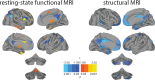
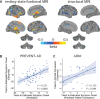
See Tijms and Visser (doi:10.1093/brain/awy113) for a scientific commentary on this article.Alzheimer's disease is preceded by a lengthy 'preclinical' stage spanning many years, during which subtle brain changes occur in the absence of overt cognitive symptoms. Predicting when the onset of disease symptoms will occur is an unsolved challenge in individuals with sporadic Alzheimer's disease. In individuals with autosomal dominant genetic Alzheimer's disease, the age of symptom onset is similar across generations, allowing the prediction of individual onset times with some accuracy. We extend this concept to persons with a parental history of sporadic Alzheimer's disease to test whether an individual's symptom onset age can be informed by the onset age of their affected parent, and whether this estimated onset age can be predicted using only MRI. Structural and functional MRIs were acquired from 255 ageing cognitively healthy subjects with a parental history of sporadic Alzheimer's disease from the PREVENT-AD cohort. Years to estimated symptom onset was calculated as participant age minus age of parental symptom onset. Grey matter volume was extracted from T1-weighted images and whole-brain resting state functional connectivity was evaluated using degree count. Both modalities were summarized using a 444-region cortical-subcortical atlas. The entire sample was divided into training (n = 138) and testing (n = 68) sets. Within the training set, individuals closer to or beyond their parent's symptom onset demonstrated reduced grey matter volume and altered functional connectivity, specifically in regions known to be vulnerable in Alzheimer's disease. Machine learning was used to identify a weighted set of imaging features trained to predict years to estimated symptom onset. This feature set alone significantly predicted years to estimated symptom onset in the unseen testing data. This model, using only neuroimaging features, significantly outperformed a similar model instead trained with cognitive, genetic, imaging and demographic features used in a traditional clinical setting. We next tested if these brain properties could be generalized to predict time to clinical progression in a subgroup of 26 individuals from the Alzheimer's Disease Neuroimaging Initiative, who eventually converted either to mild cognitive impairment or to Alzheimer's dementia. The feature set trained on years to estimated symptom onset in the PREVENT-AD predicted variance in time to clinical conversion in this separate longitudinal dataset. Adjusting for participant age did not impact any of the results. These findings demonstrate that years to estimated symptom onset or similar measures can be predicted from brain features and may help estimate presymptomatic disease progression in at-risk individuals.
Figures


Comment in
-
Neuroimaging model predicts time of symptom onset in sporadic AD.Nat Rev Neurol. 2018 Jun;14(6):315. doi: 10.1038/s41582-018-0011-1. Nat Rev Neurol. 2018. PMID: 29765131 No abstract available.
-
Chasing the start of sporadic Alzheimer's disease running in families.Brain. 2018 Jun 1;141(6):1589-1591. doi: 10.1093/brain/awy113. Brain. 2018. PMID: 29800474 No abstract available.
References
-
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

