Distinct gene networks modulate floral induction of autonomous maize and photoperiod-dependent teosinte
- PMID: 29688423
- PMCID: PMC5972621
- DOI: 10.1093/jxb/ery110
Distinct gene networks modulate floral induction of autonomous maize and photoperiod-dependent teosinte
Abstract
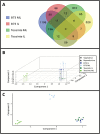
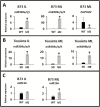
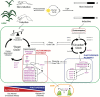
Temperate maize was domesticated from its tropical ancestor, teosinte. Whereas temperate maize is an autonomous day-neutral plant, teosinte is an obligate short-day plant that requires uninterrupted long nights to induce flowering. Leaf-derived florigenic signals trigger reproductive growth in both teosinte and temperate maize. To study the genetic mechanisms underlying floral inductive pathways in maize and teosinte, mRNA and small RNA genome-wide expression analyses were conducted on leaf tissue from plants that were induced or not induced to flower. Transcriptome profiles reveal common differentially expressed genes during floral induction, but a comparison of candidate flowering time genes indicates that photoperiod and autonomous pathways act independently. Expression differences in teosinte are consistent with the current paradigm for photoperiod-induced flowering, where changes in circadian clock output trigger florigen production. Conversely, differentially expressed genes in temperate maize link carbon partitioning and flowering, but also show altered expression of circadian clock genes that are distinct from those altered upon photoperiodic induction in teosinte. Altered miRNA399 levels in both teosinte and maize suggest a novel common connection between flowering and phosphorus perception. These findings provide insights into the molecular mechanisms underlying a strengthened autonomous pathway that enabled maize growth throughout temperate regions.
Figures


Similar articles
-
Florigen-Encoding Genes of Day-Neutral and Photoperiod-Sensitive Maize Are Regulated by Different Chromatin Modifications at the Floral Transition.Plant Physiol. 2015 Aug;168(4):1351-63. doi: 10.1104/pp.15.00535. Epub 2015 Jun 17. Plant Physiol. 2015. PMID: 26084920 Free PMC article.
-
ZCN8 encodes a potential orthologue of Arabidopsis FT florigen that integrates both endogenous and photoperiod flowering signals in maize.J Exp Bot. 2011 Oct;62(14):4833-42. doi: 10.1093/jxb/err129. Epub 2011 Jul 5. J Exp Bot. 2011. PMID: 21730358 Free PMC article.
-
Transcript and metabolite signature of maize source leaves suggests a link between transitory starch to sucrose balance and the autonomous floral transition.J Exp Bot. 2012 Sep;63(14):5079-92. doi: 10.1093/jxb/ers158. Epub 2012 Jul 12. J Exp Bot. 2012. PMID: 22791826 Free PMC article.
-
Photoperiodic flowering: time measurement mechanisms in leaves.Annu Rev Plant Biol. 2015;66:441-64. doi: 10.1146/annurev-arplant-043014-115555. Epub 2014 Dec 12. Annu Rev Plant Biol. 2015. PMID: 25534513 Free PMC article. Review.
-
Time to flower: interplay between photoperiod and the circadian clock.J Exp Bot. 2015 Feb;66(3):719-30. doi: 10.1093/jxb/eru441. Epub 2014 Nov 4. J Exp Bot. 2015. PMID: 25371508 Review.
Cited by
-
INDETERMINATE1-mediated expression of FT family genes is required for proper timing of flowering in Brachypodium distachyon.Proc Natl Acad Sci U S A. 2023 Nov 14;120(46):e2312052120. doi: 10.1073/pnas.2312052120. Epub 2023 Nov 7. Proc Natl Acad Sci U S A. 2023. PMID: 37934817 Free PMC article.
-
Adaptive introgression from maize has facilitated the establishment of teosinte as a noxious weed in Europe.Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25618-25627. doi: 10.1073/pnas.2006633117. Epub 2020 Sep 28. Proc Natl Acad Sci U S A. 2020. PMID: 32989136 Free PMC article.
-
Evaluation and application of tools for the identification of known microRNAs in plants.Appl Plant Sci. 2021 Apr 7;9(3):e11414. doi: 10.1002/aps3.11414. eCollection 2021 Mar. Appl Plant Sci. 2021. PMID: 33854848 Free PMC article. Review.
-
CONSTANS, a HUB for all seasons: How photoperiod pervades plant physiology regulatory circuits.Plant Cell. 2024 May 29;36(6):2086-2102. doi: 10.1093/plcell/koae090. Plant Cell. 2024. PMID: 38513610 Free PMC article. Review.
-
Variation in Maize Chlorophyll Biosynthesis Alters Plant Architecture.Plant Physiol. 2020 Sep;184(1):300-315. doi: 10.1104/pp.20.00306. Epub 2020 Jul 8. Plant Physiol. 2020. PMID: 32641472 Free PMC article.
References
-
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056. - PubMed
-
- Araki T, Kobayashi Y, Kaya H, Iwabuchi M. 1998. The flowering-time gene FT and regulation of flowering in Arabidopsis. Journal of Plant Research 111, 277–281.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

