Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial
- PMID: 29688502
- PMCID: PMC5991220
- DOI: 10.1210/jc.2018-00070
Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial
Abstract
Context: Combination therapy with insulin and glucagon-like peptide-1 receptor agonists (GLP-1RAs) is important for treating type 2 diabetes (T2D). This trial assesses the efficacy and safety of semaglutide, a GLP-1RA, as an add-on to basal insulin.
Objective: To demonstrate the superiority of semaglutide vs placebo on glycemic control as an add-on to basal insulin in patients with T2D.
Design: Phase 3a, double-blind, placebo-controlled, 30-week trial.
Setting: This study included 90 sites in five countries.
Patients: We studied 397 patients with uncontrolled T2D receiving stable therapy with basal insulin with or without metformin.
Interventions: Subcutaneous semaglutide 0.5 or 1.0 mg once weekly or volume-matched placebo.
Main outcome measures: Primary endpoint was change in glycated Hb (HbA1c) from baseline to week 30. Confirmatory secondary endpoint was change in body weight from baseline to week 30.
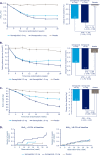
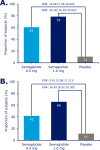
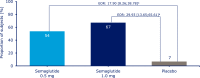
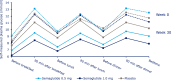
Results: At week 30, mean HbA1c reductions [mean baseline value, 8.4% (67.9 mmol/mol)] with semaglutide 0.5 and 1.0 mg were 1.4% (15.8 mmol/mol) and 1.8% (20.2 mmol/mol) vs 0.1% (1.0 mmol/mol) with placebo [estimated treatment difference (ETD) vs placebo, -1.35 (14.8 mmol/mol); 95% CI, -1.61 to -1.10 and ETD, -1.75% (19.2 mmol/mol); 95% CI, -2.01 to -1.50; both P < 0.0001]. Severe or blood glucose-confirmed hypoglycemic episodes were reported in 11 patients (17 events) and 14 patients (25 events) with semaglutide 0.5 and 1.0 mg, respectively, vs seven patients (13 events) with placebo (estimated rate ratio vs placebo, 2.08; 95% CI, 0.67 to 6.51 and estimated rate ratio vs placebo, 2.41; 95% CI, 0.84 to 6.96 for 0.5 and 1.0 mg; both P = nonsignificant). Mean body weight decreased with semaglutide 0.5 and 1.0 mg vs placebo from baseline to end of treatment: 3.7, 6.4, and 1.4 kg (ETD, -2.31; 95% CI, -3.33 to -1.29 and ETD, -5.06; 95% CI, -6.08 to -4.04 kg; both P < 0.0001). Premature treatment discontinuation due to adverse events was higher for semaglutide 0.5 and 1.0 mg vs placebo (4.5%, 6.1%, and 0.8%), mainly due to gastrointestinal disorders.
Conclusions: Semaglutide, added to basal insulin, significantly reduced HbA1c and body weight in patients with uncontrolled T2D vs placebo.
Trial registration: ClinicalTrials.gov NCT02305381.
Figures
References
-
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. - PubMed
-
- Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract. 2011;65(3):314–322. - PubMed
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. - PubMed
-
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

