Pharmacokinetics of Upadacitinib With the Clinical Regimens of the Extended-Release Formulation Utilized in Rheumatoid Arthritis Phase 3 Trials
- PMID: 29688617
- PMCID: PMC6585649
- DOI: 10.1002/cpdd.462
Pharmacokinetics of Upadacitinib With the Clinical Regimens of the Extended-Release Formulation Utilized in Rheumatoid Arthritis Phase 3 Trials
Abstract
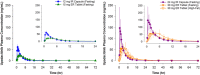
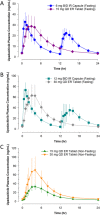
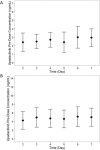
Upadacitinib is a Janus kinase 1 inhibitor under development for the treatment of several inflammatory disorders including rheumatoid arthritis (RA). Upadacitinib was administered in the phase 2 RA trials primarily as twice-daily regimens of an immediate-release (IR) formulation. The upadacitinib extended-release (ER) formulation was developed to enable once-daily dosing. In the present study, upadacitinib pharmacokinetics were characterized after the administration of single and multiple once-daily doses of the ER formulation in healthy subjects relative to single and multiple twice-daily doses of the IR formulation. Increase in upadacitinib exposure was dose-proportional over the evaluated 15- to 30-mg ER dose range. Single 15- and 30-mg ER doses provided equivalent AUC0-inf compared with single 12- and 24-mg IR doses, respectively. A high-fat breakfast increased upadacitinib ER Cmax and AUC0-inf by only 20% and 17%, respectively, relative to fasting conditions. The median time to peak plasma concentrations was 2 to 4 hours for the ER formulation, and steady state was achieved by day 4 of once-daily dosing. Doses of 15 and 30 mg once daily using the ER formulation provided equivalent AUC0-24 , comparable Cmax and Cmin , and a fluctuation index over a 24-hour period at steady state similar to 6 and 12 mg twice daily, respectively, using the IR formulation. These results supported the use of upadacitinib 15- and 30-mg doses of the ER formulation in the phase 3 trials in RA.
Keywords: ABT-494; JAK1 inhibitors; extended-release formulation; pharmacokinetics; rheumatoid arthritis; upadacitinib.
© 2018 The Authors. Clinical Pharmacology in Drug Development Published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Conflict of interest statement
Mohamed‐Eslam F. Mohamed, Jiewei Zeng, Patrick J. Marroum, In‐Ho Song, and Ahmed A. Othman are employees of AbbVie Inc. and may hold AbbVie stock and/or stock options. Medical writing support was provided by Therese Stickler, a freelance writer under contract with AbbVie.
Figures

Similar articles
-
Population Pharmacokinetics of Upadacitinib Using the Immediate-Release and Extended-Release Formulations in Healthy Subjects and Subjects with Rheumatoid Arthritis: Analyses of Phase I-III Clinical Trials.Clin Pharmacokinet. 2019 Aug;58(8):1045-1058. doi: 10.1007/s40262-019-00739-3. Clin Pharmacokinet. 2019. PMID: 30945116 Free PMC article. Clinical Trial.
-
Pharmacokinetics of Upadacitinib in Healthy Subjects and Subjects With Rheumatoid Arthritis, Crohn's Disease, Ulcerative Colitis, or Atopic Dermatitis: Population Analyses of Phase 1 and 2 Clinical Trials.J Clin Pharmacol. 2020 Apr;60(4):528-539. doi: 10.1002/jcph.1550. Epub 2019 Nov 7. J Clin Pharmacol. 2020. PMID: 31701537
-
Pharmacokinetics, Safety and Tolerability of ABT-494, a Novel Selective JAK 1 Inhibitor, in Healthy Volunteers and Subjects with Rheumatoid Arthritis.Clin Pharmacokinet. 2016 Dec;55(12):1547-1558. doi: 10.1007/s40262-016-0419-y. Clin Pharmacokinet. 2016. PMID: 27272171 Clinical Trial.
-
Population Pharmacokinetics of Upadacitinib in Healthy Subjects and Subjects with Rheumatoid Arthritis: Analyses of Phase I and II Clinical Trials.Clin Pharmacokinet. 2018 Aug;57(8):977-988. doi: 10.1007/s40262-017-0605-6. Clin Pharmacokinet. 2018. PMID: 29076110 Free PMC article. Review.
-
A review of upadacitinib in rheumatoid arthritis.Mod Rheumatol. 2020 Sep;30(5):779-787. doi: 10.1080/14397595.2020.1782049. Epub 2020 Jul 13. Mod Rheumatol. 2020. PMID: 32530345 Review.
Cited by
-
Efficacy and Safety of Upadacitinib Monotherapy in Methotrexate-Naive Patients With Moderately-to-Severely Active Rheumatoid Arthritis (SELECT-EARLY): A Multicenter, Multi-Country, Randomized, Double-Blind, Active Comparator-Controlled Trial.Arthritis Rheumatol. 2020 Oct;72(10):1607-1620. doi: 10.1002/art.41384. Epub 2020 Sep 8. Arthritis Rheumatol. 2020. PMID: 32638504 Free PMC article. Clinical Trial.
-
Characterization of the Effect of Renal Impairment on Upadacitinib Pharmacokinetics.J Clin Pharmacol. 2019 Jun;59(6):856-862. doi: 10.1002/jcph.1375. Epub 2019 Jan 11. J Clin Pharmacol. 2019. PMID: 30633369 Free PMC article.
-
Effect of Upadacitinib on the Pharmacokinetics of Rosuvastatin or Atorvastatin in Healthy Subjects.Clin Pharmacol Drug Dev. 2021 Nov;10(11):1335-1344. doi: 10.1002/cpdd.957. Epub 2021 Jun 9. Clin Pharmacol Drug Dev. 2021. PMID: 34109764 Free PMC article. Clinical Trial.
-
Upadacitinib Population Pharmacokinetics and Exposure-Response Relationships in Ulcerative Colitis Patients.Clin Pharmacokinet. 2023 Jan;62(1):101-112. doi: 10.1007/s40262-022-01191-6. Epub 2022 Dec 26. Clin Pharmacokinet. 2023. PMID: 36571701 Free PMC article.
-
Development of In Vitro-In Vivo Correlation for Upadacitinib Extended-Release Tablet Formulation.AAPS J. 2019 Oct 25;21(6):108. doi: 10.1208/s12248-019-0378-y. AAPS J. 2019. PMID: 31654328 Free PMC article. Clinical Trial.
References
-
- AbbVie . A study comparing ABT‐494 to placebo in subjects with rheumatoid arthritis on a stable dose of conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs) who have an inadequate response to csDMARDs alone (SELECT‐NEXT) [ClinicalTrials.gov identifier NCT02675426]. Accessed July 13, 2017.
-
- AbbVie . A multicenter, randomized, double‐blind, placebo‐controlled study of ABT‐494 for the induction of symptomatic and endoscopic remission in subjects with moderately to severely active Crohn's disease who have inadequately responded to or are intolerant to anti‐TNF therapy (Celest Study) [ClinicalTrials.gov identifier NCT02365649]. Accessed July 13, 2017.
-
- AbbVie . A study to evaluate the safety and efficacy of ABT‐494 for induction and maintenance therapy in subjects with moderately to severely active ulcerative colitis [ClinicalTrials.gov identifier NCT02819635]. Accessed July 13, 2017.
-
- AbbVie . A study to evaluate ABT‐494 in adult subjects with moderate to severe atopic dermatitis [ClinicalTrials.gov identifier NCT02925117]. Accessed July 13, 2017.
-
- AbbVie . A study comparing ABT‐494 to placebo and to adalimumab in participants with psoriatic arthritis who have an inadequate response to at least one non‐biologic disease modifying anti‐rheumatic drug (SELECT ‐ PsA 1) [ClinicalTrials.gov identifier NCT03104400]. Accessed July 13, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

